Abstract
Therapy-related myelodysplastic syndrome (t-MDS) is a well-known complication of conventional chemoradiotherapy used to treat a variety of hematologic malignancies as well as solid tumors. Because of poor prognosis of t-MDS when treated with conventional therapy, allogeneic hematopoietic stem cell transplantation (HSCT) is usually recommended with encouraging results reported.
Herein, we retrospectively analyzed outcomes in of 309 adult patients with t-MDS (n =101) and de novo MDS (n=208) receiving allogeneic HSCT from 2005 to 2015 at MD Anderson Cancer Center and compared their outcomes adjusted for disease characteristics.
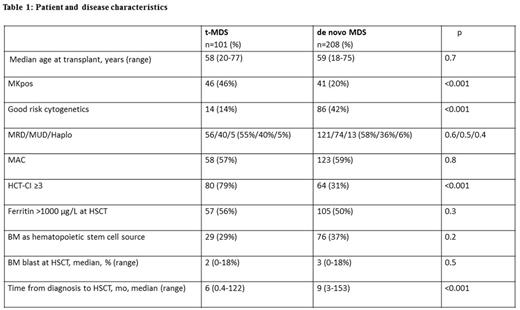
The study cohort had median age of 59 (range, 18-77) at HSCT. Histological subtype at diagnosis was RAEB-1 and -2 in 120 (39%) and CMML in 30 (10%) patients. When cytogenetics were classified by monosomal karyotype (MK) defined by Breems et al., 87 patients (27%) had MK-positive (MKpos), 99 patients (32%) MK-negative (MKneg) and 100 patients (32%) good risk cytogenetics (including normal karyotype (n=88) and del(5q), del(12p), del(20q) (n=12)). Donors were matched related donor (MRD, n=177), matched unrelated donor (MUD, n=114) or haploidentical donor (n=18). Most patients received a myeloablative conditioning regimen (n=181, 59%). Hematopoietic stem cell comorbidity index (HCT-CI) was ≥3 in 144 (47%) patients.
Among t-MDS and de novo MDS; median age, donor type and conditioning intensity were not different (Table 1). Compared with de novo MDS, t-MDS patients had more MKpos (20% vs. 46%, p<0.001) less good risk cytogenetics (38% vs. 10%, p<0.001) and more HCT-CI ≥3 (31% vs. 79%, p<0.001). The median time from diagnosis to HSCT was shorter in t-MDS (6 mo. vs. 9 mo., p<0.001).
The median follow up of 139 survivors was 37 (range, 3-125) months. Outcome analyses were stratified for t-MDS and de novo MDS patients due to different disease characteristics observed.
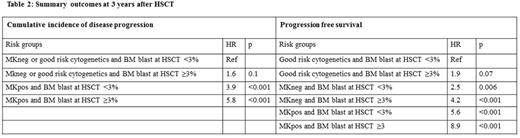
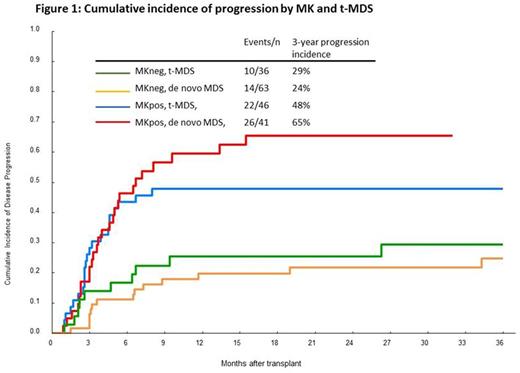
Univariate analyses showed that MKpos was associated with a poor prognosis for the cumulative incidence of progression both in t-MDS and de novo MDS compared with good cytogenetics (HR=4.3, p=0.05, HR=6.9, p<0.001, respectively) (Figure 1). BM blast count ≥3% at HSCT was also associated with increased incidence of progression in both groups (HR=1.5, p=0.2, HR=1.9, p=0.02, respectively). This enabled us to analyze t-MDS and de novo MDS together for multivariate regressions which revealed that MKpos patients with BM blast count ≥3% had the highest risk for disease progression compared with patients without MKpos and with BM blast count <3% (HR=5.8) (Table 2). Patients with MKpos but with BM blast count <3% also had higher incidence of progression (HR=3.9). The outcomes by risk groups for progression was comparable for t-MDS and de novo MDS (Data not presented).
For progression free survival (PFS), univariate analyses revealed that MKpos had poor prognosis for both in t-MDS and de novo MDS compared with good-risk cytogenetics (HR=2.9, p=0.025 and HR=8.4, p<0.001, respectively). MKneg were also associated with inferior PFS compared with good-risk cytogenetics in both t-MDS and de novo MDS (HR=2.1, p=0.1 and HR=2.3, p=0.001, respectively). BM blast count ≥3% also decreased PFS both in t-MDS and de novo MDS for PFS (HR=1.6, p=0.07 and HR=1.8, p=0.004, respectively). When t-MDS and de novo MDS were analyzed together for multivariate regressions, we found that MKpos patients with BM blast count ≥3% had lowest PFS while patients good risk cytogenetics had the best outcomes independent of their BM blast count (Table 2). There was no impact of t-MDS on PFS and similar to progression, the outcomes were not inferior in t-MDS (Data not presented).
In conclusion, although t-MDS has been felt to have a relatively poor prognosis after HSCT, our results suggest that t-MDS has comparable outcomes with de novo MDS after allogeneic HSCT when adjusted for disease characteristics. Patients with MKpos in t-MDS and de novo MDS have very high incidence of disease progression and low PFS and represent a target population for innovative transplant strategies.
Ciurea:Cyto-Sen Therapeutics: Equity Ownership; Spectrum Pharmaceuticals: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal