Abstract
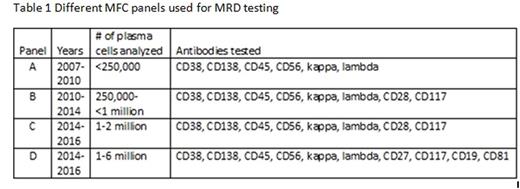
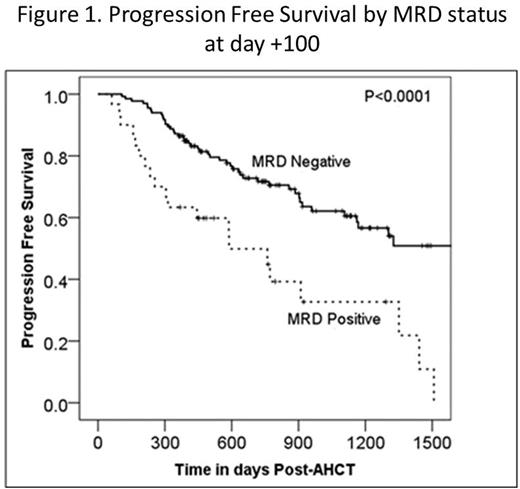
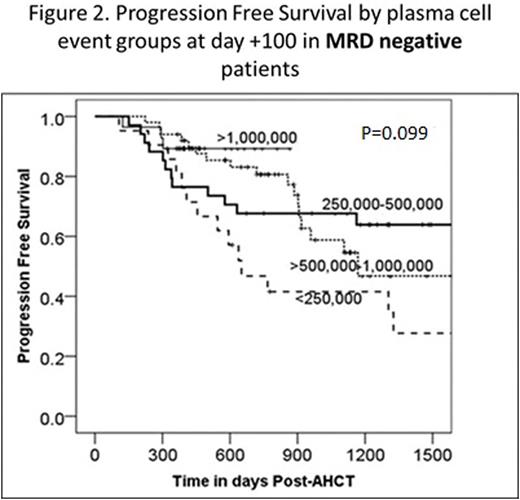
Minimal residual disease (MRD) after autologous hematopoietic cell transplant (AHCT) in multiple myeloma (MM) has been shown to be an important predictor of clinical outcomes, suggesting that MRD negativity may be a new goal of therapy. Multiparametric flow cytometry (MFC) is a commonly used method for MRD assessment, however this technique is still evolving and efforts are underway to standardize this testing. The key factors which enable detection of residual malignant plasma cells by MFC remain an area of active investigation. We performed a retrospective review of 172 consecutive MM patients who received AHCT between 10/1/2007 and 5/31/2015 at our institution and had undergone MRD assessment by MFC at day +100 post-AHCT. Day +100 post-AHCT response was determined using the International Myeloma Working Group (IMWG) Uniform Response Criteria (URC) and was correlated with MRD assessment as well as progression free survival (PFS) and overall survival (OS). Data were collected on the specific MFC panel utilized, including the epitopes analyzed and the total plasma cell number (PCN) counted (normal and malignant PC). These variables were correlated with clinical outcomes including day +100 MM response, PFS and OS. Of 172 patients, 30 were MRD-positive, 133 MRD-negative, and 9 were equivocal at day +100 post-AHCT, the latter of which were excluded from further analyses. Day+100 MRD-negative status by MM response was: 31/37(84%) for VGPR, 35/41 (85%) for CR, and 42/42 (100%) for sCR. Patients who achieved a CR or sCR had improved PFS and OS rates compared with patients who achieved ≤VGPR: 3-year PFS: 61% (95% CI 49-74%) vs 46% (95% CI 32-59%), P=0.03; 3-year OS: 96% (95% CI 91-100%) vs 69% (95% CI 56-81%), P=0.005)). Patients with MRD-negative disease at day +100 post-AHCT had significantly superior PFS and OS compared to those with MRD-positive disease: 3-yr PFS 62% (95% CI 52-72%) vs 33% (95% CI 12-53%), P <0.0001) (Figure 1); 3-year OS 85% (95% CI 78-93%) vs 64% (95% CI 44-85%), P=0.004). There was no association between MRD status and age (<60 vs ≥60 years), sex, race (white vs other), performance status (KPS ≤80 vs ≥90), or subsequent transplant (P>0.1). The details of the four different MRD MFC panels are shown in Table 1. Panels C and D were compared, at a similar PCN level, but different epitopes tested, and found no significant difference in PFS or OS. Further analysis of PCN within the MRD-negative cohort revealed a trend towards improved 3-yr PFS rates with increasing numbers of PCN analyzed: 42% (95% CI 20-63%) for PCN<250,000, 68% (95% CI 52-83%) for PCN=250,000-500,000, 59% (95% CI 42-76%) for PCN >500,000-1,000,000 and 89% (78-100%) for PCN>1,000,000 (P=0.099) (Figure 2). The 3-yr OS rates for MRD-negative patients were higher for increasing PCNs analyzed, but the PCN categories were not statistically significantly different: 74% (95% CI 54-94%) for PCN<250,000, 88% (95% CI 77-99%) for PCN=250,000-500,000, 85% (95% CI 73-98%) for PCN >500,000-1,000,000 and 100% for PCN>1,000,000 (P=0.2). Sensitivity analysis revealed similar trends when a cut-off of above or below 500,000 or 1,000,000 was used. Our results confirm that achievement of MFC MRD negativity at day +100 post-AHCT is associated with improved PFS and OS. Factors such as the long-half lives of immunoglobulins, the quality of the bone marrow aspirate obtained, and the presence of occult extramedullary disease may account for the patients who were MRD negative but did not achieve a CR at day +100 post AHCT by IMWG URC. MRD assessment by MFC at our institution has evolved over time to include higher numbers of acquired and analyzed events. Notably, there was a trend towards improved outcomes with greater numbers of plasma cells analyzed, suggesting that continued development of MRD assessment by MFC should focus on increasing PCN analyzed in order to improve detection of residual MM clones.
Hahn:Novartis: Equity Ownership; NIH: Research Funding. McCarthy:The Binding Site: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gamida Cell: Honoraria, Membership on an entity's Board of Directors or advisory committees. Holstein:Millennium: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal