Abstract
Background: Necessity of maintenance therapy (MT) in patients with multiple myeloma (MM) after auto-HSCT continues to be a matter of debate in the community. To ensure it's proper application, it is important to set clear criteria when it is an appropriate type of treatment. MRD status of the patient after auto-HSCT could be such a criteria, than can be used to stratify the risk of relapse and justify the MT.
Aims: To assess the efficacy of MT in patients with MM achieving a complete response after auto-HSCT accounting on whether or not they have minimal residual disease.
Patients and Methods: Fifty-two patients with MM (19 male and 33 female, 24 to 66 years old - median, 54 years) who had achieved a complete response after auto-HSCT were enrolled in a prospective randomized clinical study in the period from January 2014 to February 2016. Subjects were at the following stages of the disease (ISS): 11 at Stage I, 23 at Stage II, and 18 at Stage III. The remission induction protocol included: bortezomib-containing regimens (VCD, PAD) for all 52 patients, immunomodulatory agents for 8, and bendamustine for 2. Distribution of patients by type of anti-tumor responses (according to the International Myeloma Working Group classification criteria) after induction was as follows: complete response in 26 patients (50 %), very good partial response in 12 subjects (23 %), and partial response in 5 individuals (27 %). Single auto-HSCT was used in 36 subjects and tandem auto-HSCT in 16. At 100 days post auto-HSCT, the MRD status was assessed by means of bone marrow examination using six-color Flow Cytometry with a panel of antibodies to the following antigens: CD38, CD138, CD45, CD56, CD117, and CD19. Tests yielding less than 50 clonal plasma cells in 500,000 white blood cells (< 0.01 %, Limit of Detection: 10-4) were reported as an MRD-negative status. A stringent complete response after auto-HSCT had been achieved in 25 patients (48 %), while an MRD-positive status was detected in 27 subjects (52 %). At 100 days post auto-HSCT, MRD-negative patients were randomized into two arms: 14 subjects were given bortezomib MT (1.3 mg/m2 s.c., one dose every two weeks for one year), and 11 patients were not administered M"; one female patient was subsequently withdrawn from maintenance therapy due to drug toxicity. Some of the MRD-positive subjects (19) received bortezomib MT according to the aforementioned regimen, while the others (8 patients) did not receive MT. The follow-up period from the time of MRD detection ranged from 5 months to 27 months (median 14 months). Two deaths (unrelated to the underlying condition) were registered in the course of the trial.
Relapse-free survival (RFS) rate was selected as the efficacy endpoint for MM patients. Survival curves were constructed using the Kaplan-Meier method and a log-rank test was used to compare the survival estimates between two groups. The compared groups were overall balanced for known prognostic factors. Statistical analysis was done using SAS 9.4.
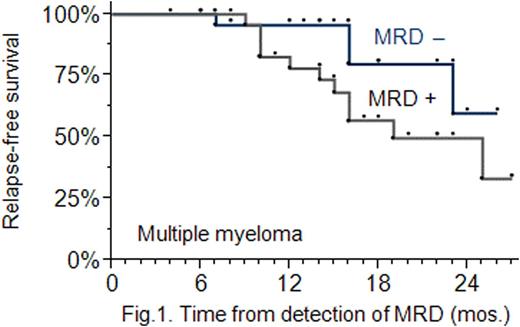
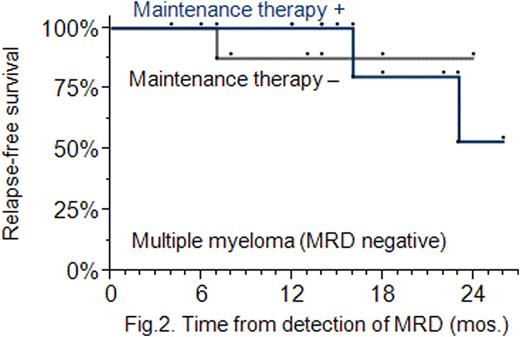
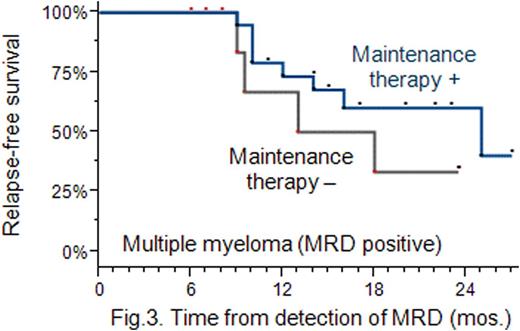
Results: MRD-positive patients were found to have a lower 2-year RFS rate compared with MRD-negative patients: 49 % vs 60 % (P=0.056); the presence of MRD increases the risk of relapse (Hazard Ratio: 1.7, 95 % CI: 1.3 − 3.4) (Fig. 1). MT administered in MRD-negative patients did not increase their RFS: the 2-year RFS rates were found to be approximately the same in the MT and no MT arms (P=0.46), and equal to 84 % (Fig. 2). MT used in MRD-positive patients did increase their RFS: the 2-year RFS rate was higher in patients treated with MT compared with the no MT treated: 43 % vs 35 % (P=0.076) (Fig. 3). Following completion of MT, 8 out of 19 MRD-positive patients (42 %) achieved a better antitumour response, i. e. a stringent complete response.
Conclusion: The reported study demonstrated that assessment of the MRD status provides an effective criterion for risk stratification in patients who have received auto-HSCT and are considered for MT. Maintenance therapy appears to be most appropriate in patients found to be MRD-positive after auto-HSCT, while for MRD-negative patients carrying out MT is inappropriate because it does not result in significant effect, but is associated with toxic complications.
This study was approved by the local ethics committee.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal