Abstract
Background
The pulmonary function test (PFT) is an integral part of the assessment of patients prior to hematopoietic cell transplantation (HCT). The HCT comorbidity index (HCT-CI) is a routinely used scoring system using PFTs to determine patient outcomes post HCT. FEV1 and DLCO are used as determinants distinguishing between moderate (DLCO or FEV1 66-80%) and severe dysfunction (DLCO or FEV1 ≤ 65%). While there is a supportive evidence of its use in HCT, there is a debate on stratification of the current threshold of PFTs. Our study attempted to analyze the prognostic impact of each parameter of PFT on outcomes including overall survival (OS), relapse and non-relapse mortality (NRM) including FEV1, FVC, DLCO, FEF50%/75%, FRC, RV as well as TLC.
Methods
A retrospective review was conducted to analyze pre-transplant PFT values with respect to transplant outcomes in 605 patients receiving allogeneic HCT at Princess Margaret Cancer Centre from 2004 to 2013. PFT results were collected within 30 days prior to HCT. PFT parameters include % predicted FEV1, DLCO, TLC, RV, RV/TLC, FRC, FVC, FEV1/FVC, FEF50% and FEF75%. The main outcomes include OS, cumulative incidence of relapse (CIR) and NRM. The cut-off values in each PFT parameters with respect to OS were calculated using binary recursive partitioning (rpart) method provided by rpart package using R. Each PFT parameter was analyzed for correlation with other PFT parameters using Pearson's correlation test. All the statistical tests were done using EZR and SPSS. OS analysis was done using Kaplan-Meier method, and OS was compared according to the new cutoff derived from rpart and according to the cutoff used in the HCT-CI score. Then prognostic stratification power was compared between these two cutoffs. For the multivariable analysis, all variables were included in the model with the exception of those already included in the HCT-CI score. For the outcome of OS, Cox prop ortional hazard regression model was adopted. Six clinical variables were included in the multivariate analysis including: pre-transplant FEV1 <81%, conditioning regimen (reduced intensity vs myeloablative), age ≥60 yrs (vs < 60 yrs), disease risk index (DRI) (high/very high risk vs intermediate vs low risk), GVHD prophylaxis (without or with T-Cell Depletion (TCD)), and HLA mismatch vs matched.
Results
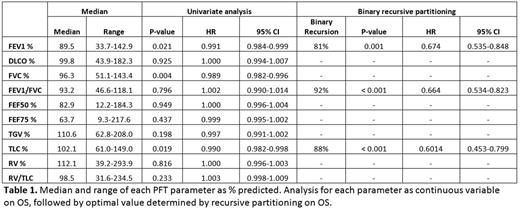
Out of 605 patients, OS rate was 46% at 3 years. NRM was 35.6% and relapse rate was 21.0% at 3 years. For correlation among PFT parameters, FEV1 was significantly associated with TLC (r=0.51), FVC (r=0.79), FEF75 (r=0.58) and FEF50 (r=0.68), and was chosen as a surrogate of OS for further analysis. Univariate analysis for the PFT parameters expressed as continuous variables revealed that OS was associated with FEV1, FVC and TLC, while not with other parameters (i.e. DLCO, FEF50%, FEF70%, FRC or RV). Using recursive partitioning, the optimal value for the best stratification of OS was found: FEV1 at 81%, FEV1/FVC at 92%, and TLC as 88%. The OS rate at 3 years in the group with FEV1 ≥81% was 49.9%, versus FEV1 <81%, 36.6% (p=0.001, HR 0.674). Meanwhile, the group with FEV1 ≥80% showed an OS of 49.2% at 3 years, while with FEV1 between 66-80% and ≤65% showed an OS of 38.1% and 33.5% at 3 years, respectively. (p=0.003, HR 0.774). We then compared the risk stratification power of the new FEV1 cutoff derived from rpart with the cutoff used in the HCT-CI score (65% and 80%). Using C-statistic, it was found that the new cutoff from rpart showed better risk stratification compared to that form HCT-CI score (P<0.01, C-statistic 2.4279). Multivariate analysis confirmed that pre-transplant FEV1 <81% is an independent prognostic factor for OS. The patients with pre-transplant FEV1 <81% showed 48% higher risk of death compared to those with higher FEV1 (p=0.001; HR 1.478). The other prognostic factors included HLA mismatch (p=0.001; HR 1.817]), and DRI (overall p=0.03; p=0.007 between low vs intermediate/high/very high risk).
Conclusions
1. FEV1 at 81% is a significant and reliable parameter amongst all the PFT parameters affecting HCT outcomes.
2. DLCO does not predict long-term HCT outcomes in our cohort.
3. Compared to FEV1 cutoff per HCT-CI scoring system, the new cutoff of 81% FEV1 provides better risk stratification power for long-term outcomes after allogeneic HCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal