Abstract

Introduction
Blinatumomab, a bispecific T-cell engager antibody construct, binds specifically to CD19 (B cells) and CD3 (T cells) to facilitate lysis of CD19-positive target B-lineage cells. Based on a single-arm phase 2 study, blinatumomab received approvals in the US (accelerated) and EU (conditional) for the treatment of Philadelphia chromosome-negative (Ph-) relapsed/refractory (r/r) B-cell precursor acute lymphoblastic leukemia (BCP-ALL). In the phase 3, randomized, open-label TOWER study, blinatumomab immunotherapy significantly improved overall survival (OS) as compared to SOC chemotherapy in adult patients with Ph- r/r BCP-ALL. This abstract reports the impact of blinatumomab compared with SOC on HRQoL using data from TOWER.
Methods
Patients (n=405) with r/r BCP-ALL were randomized 2:1 to receive either blinatumomab (n=271) or SOC chemotherapy (n=134). Patients in remission after 2 induction cycles were eligible to continue therapy. A 12-month maintenance phase was allowed for patients who received up to 3 consolidation cycles and continued with a bone marrow response. HRQoL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) on day 1 (baseline), day 8, day 15, day 29 in each cycle of investigational therapy and safety follow up. The HRQoL analyses included subjects with baseline and ≥1 post-baseline result of any EORTC QLQ-C30 multi-item scale or single-item measure. The change from baseline was summarized by visit and by treatment arm. Time-to-deterioration (TTD) analyses were conducted to determine the treatment effect based on timing from the initiation of treatment to a 10-point deterioration from baseline in HRQoL using EORTC QLQ-C30 or time to an event free survival (EFS) event. EFS was defined as day 1 for subjects who never reached hematologic response, and as time to relapse following response or death for those who reached hematologic response.
Results
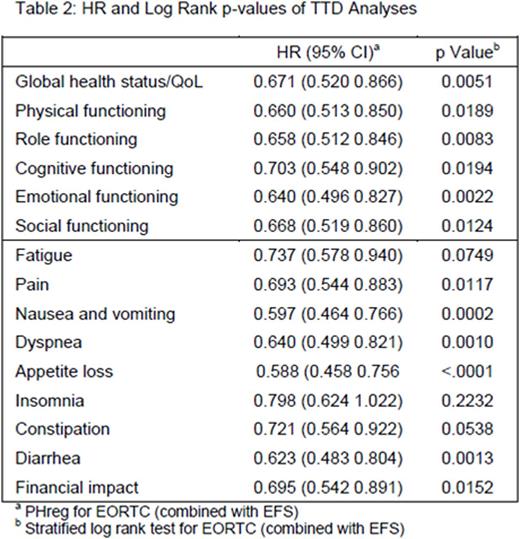
Of the 376 patients who received at least 1 dose of study drug, 342 patients had baseline and ≥1 post-baseline HRQoL results. Mean pre-treatment baseline scores were balanced between the blinatumomab (n=247) and SOC (n=95) groups. In general, patients receiving blinatumomab had better post-treatment HRQoL versus SOC across all multi-item scales or single-item measures based on visual inspection (Table1). As early as day 8 of cycle 1 in the SOC arm, mean changes from baseline suggested worsening in HRQoL in almost all subscales/single items, whereas in blinatumomab arm, mean changes from baseline suggested improvement in HRQoL in almost all subscales (Table 1). The hazard ratios (HRs) from the TTD analyses ranged from 0.588 to 0.798 in favor of blinatumomab, with the upper bounds to the 95% CI <1.0 across all subscales and single items except insomnia (Table 2).
Conclusions
Blinatumomab delayed the time to clinically meaningful deterioration in HRQoL or EFS event versus SOC across almost all functional domains and symptoms relevant to oncology patients. Moreover, HRQoL benefits with blinatumomab relative to SOC could be observed as early as 8 days after treatment initiation. The benefits in HRQoL, in addition to the significant improvement in efficacy, including OS, and acceptable safety profile of blinatumomab versus SOC, support a favorable benefit-risk profile of blinatumomab in patients with Ph- r/r BCP-ALL. Limitations of the analysis include potential bias due to different cycle lengths between blinatumomab and SOC regimens (6-week cycle for blinatumomab vs variable cycle lengths for SOC) and missing data.
Topp:Jazz: Consultancy; Roche: Honoraria, Other: travel; Pfizer: Consultancy, Other: Travel; Boehringer: Consultancy, Other: Travel, Research Funding; Amgen: Consultancy, Honoraria, Other: travel, Speakers Bureau. Zimmerman:Amgen Inc.: Employment, Equity Ownership. Dombret:Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Maertens:Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Merck Sharp & Dohme: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Astellas: Consultancy, Speakers Bureau; Amgen: Consultancy. Schuh:Amgen: Membership on an entity's Board of Directors or advisory committees. Franklin:Amgen: Employment, Other: Stocks. Nie:Amgen: Employment. Cong:Amgen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal