Abstract
Protecting from lethal graft-versus-host disease (GVHD) while preserving graft-versus-tumor effects by adoptive immunotherapy would be an ideal goal in allogeneic hematopoietic stem cell transplantation (HSCT), especially using third party derived cells, because the use of third party cells would open a huge source of availability and feasibility across major histocompatibility barriers. We have demonstrated that third party cytokine-induced killer (CIK) cells protected from murine lethal GVHD with faster hematological recovery, which are ex vivo-expanded T lymphocytes expressing both natural killer and T-cell markers with strong cytotoxic activity both against autologous and allogeneic tumor cells. However the mechanism responsible for ameliorating acute GVHD by third party CIK cells are still unknown.
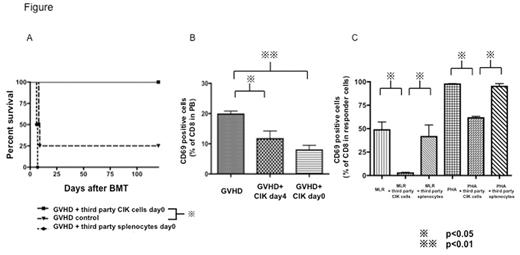
First, lethally irradiated Balb/c (H-2d) mice were given C3H (H-2k) bone marrow cells with C3H whole splenocytes to induce lethal GVHD with/without third party (C57/BL6 (H-2b)) CIK cells or third party whole splenocytes on day 0 or day4. Mice receiving third party CIK cells showed much less GVHD and significant survival benefit compared those with third party splenocytes as shown below (p<0.05). On day7 after BMT, the percentage of CD69 on CD8 T-cells in peripheral blood (PB) in the mice receiving third party CIK cells was significantly lower than those in GVHD control mice as shown below (p<0.05), meanwhile donor derived not-activated T-cells were preserved in both CD4 and CD8 T-cells. From these results, we hypothesized that third party CIK cells prevent acute GVHD by selectively depleting highly proliferative alloreactive T-cells stimulated early after transplant, while sparing naïve T-cells. To test this, we performed mixed lymphocyte reaction (MLR) by adding third party CIK cells or third party splenocytes (Stimulator: Balb/c splenocytes, Responder: C3H splenocytes, third party cells: C57/BL6). The percentage of CD69 on responder derived CD8 T-cells in the culture mixture with third party CIK cells was much less compared to that with third party splenocytes as shown below (p<0.05). We have previously shown that allogeneic CIK cells had cytotoxicity against cultured dendritic cells (DCs) by 51Cr release assay. Therefore reduced alloreaction by third party CIK cells might be due in part to the elimination of antigen presenting cells such as DCs. Next, to demonstrate that third party CIK cells could eliminate responder cells directly and then reduce the alloreaction, we performed MLR like experiments using PHA to activate T-cells strongly instead of antigen presenting cells as a stimulator. As shown below, we have demonstrated that the percentage of highly activated T-cells (CD8+CD69+) in third party CIK added plates was significantly lower compared to that in third party splenocytes added plates, indicating that third party CIK cells could have direct effects against highly activated T-cells.To further clarify the mechanisms of how third party CIK cells reduce the highly activated T-cells, we performed MLR as mentioned above with/without the neutralizing antibody against NKG2D, which is an activator receptor expressed on NK cells. Although CIK cells have cytotoxicity against tumor cells via NKG2D signaling pathway in part, adding NKG2D antibody did not show a statistical significant difference in MLR assays. It suggested that third party CIK cells could suppress alloreactive T-cells by several pathways other than NKG2D signaling pathway.
Finally, we identified the in vivo kinetics of third party CIK cells after BMT. Third party derived CIK cells could be identified as a rare subpopulation in recipient peripheral blood for some days after CIK infusion, but all of them could not be detected anymore by day 7 after infusion. Whereas third party whole splenocytes expanded in recipient peripheral blood and mice received third party splenocytes with donor BM and splenocytes died until day7 because of GVHD induced by third party splenocytes.
In conclusion, infusion of third party CIK cells has strong potential to prevent murine lethal GVHD probably due to selectively depleting highly proliferative alloreactive T-cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal