Abstract
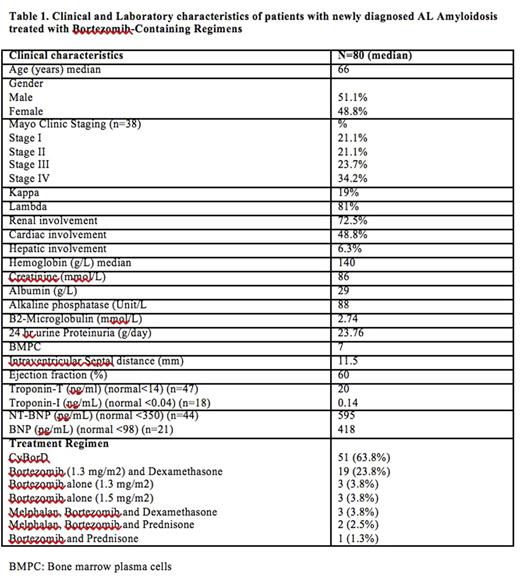
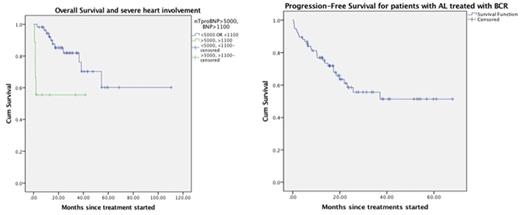
Introduction Bortezomib is a novel, dipeptide boronic acid molecule that has shown anti-tumor activity against Multiple Myeloma (MM) and more recently has been demonstrated to be efficacious in the treatment of Light-chain Amyloidosis (AL). Bortezomib-based regimens have changed the landscape of the treatment of AL Amyloidosis due to high levels of response as well as the adequate tolerance. Based on the above mentioned, we aimed to assess the role of Bortezomib-containing regimens (BCR) for the treatment of newly diagnosed AL amyloidosis. Methods 80 consecutive patients have been treated with bortezomib-containing regimens at BCCA and TBCC in Calgary, AB and Vancouver, BC, Canada from 01/2010 to 01/2016. Among these cases, 51 (63.8%) have received CyBorD, 19 (23.7%) bortezomib (1.3 mg/m2) and dexamethasone and the rest Bortezomib in different combinations (Table 1). The primary objective of the study was to assess hematological and organ response. Results Clinical characteristics are shown in Table 1. Median age at diagnosis was 66 years and 51.1% of patients were male. At the time of analysis, 56 patients are still alive and 14 patients have already progressed. A HR was seen in 65/72 evaluable patients (ORR, 90%) after a median of 6 cycles (range, 1-24). Fourteen patients (17.5%) did not have measurable disease based on light chain levels. CR (34.7%) and VGPR (26.4%) were seen in 61% of cases. Organ response was achieved in 49% of cases. Cardiac response was observed in 40% of cases. Median OS and PFS have not been reached (Estimate, 67 and 41 months, respectively). (Fig 1a) However, OS was shorter in the group with severe heart involvement (NT-pro-BNP>5000 or BNP>1100) (p=0.03) (Fig 1b). In conclusion, BCR are efficacious for the treatment of newly diagnosed AL Amyloidosis, providing with a high degree of organ and hematological responses. The impact of BCR on advanced heart dysfunction cases remains unclear but it seems that these patients still require a different approach maintaining a dismal prognosis.
Overall survival and advanced cardiac disease
Song:Janssen: Honoraria; Otsuka: Honoraria; Celgene: Honoraria, Research Funding. Neri:Celgene and Jannsen: Consultancy, Honoraria. Bahlis:Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel Expenses, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Other: Travel Expenses, Research Funding, Speakers Bureau; Onyx: Consultancy, Honoraria; BMS: Honoraria. Jimenez-Zepeda:Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Celgene, Janssen, Amgen, Onyx: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal