Abstract
Background:
Proteasome inhibition with carfilzomib (CFZ) has shown to be effective therapy in relapsed refractory multiple myeloma (MM).1 Cardiac toxicity induced by CFZ has been reported in a small subset of patients treated in phase 3 clinical trials, including congestive heart failure (CHF) in approximately 2-5% of patients. The natural history or reversibility of cardiac dysfunction with CFZ has not been fully reported. The myocardium constantly contracts in adults and thus, adequate protein metabolism may be essential; and hence, effective proteasome degradation may pose a risk for contractility.
Methods and results:
We identified 12 patients, who received CFZ at Mayo Clinic in Arizona, and had a drop in ejection fraction (EF) attributable to the use of CFZ. Medical records were reviewed for baseline cardiovascular (CV) risk factors, CV disease reversibility and mortality resulting from CFZ induced cardiac dysfunction. Patients who had a drop in EF attributed to other causes, were not included in this count.
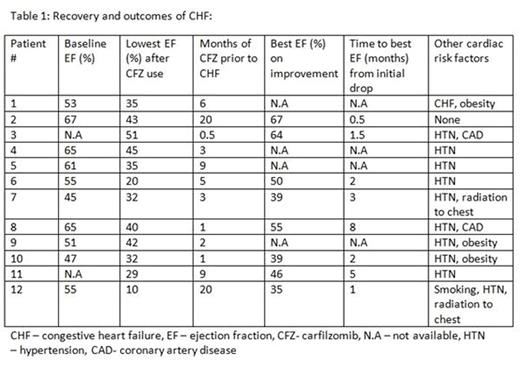
The median age at the time of initiation of therapy with CFZ was 62.5 years (range, 55-70 years), median body mass index was 27.65 (range, 21 - 40), with 4 (33%) patients being obese and 6 (50%) in the overweight range. Two (17%) patients had history of coronary artery disease while 9 (75%) had hypertension, and were on medical treatment prior to CFZ initiation. Six (46%) patients had a previous history of CHF. Two of these had symptomatic systolic dysfunction, however at the time of CFZ initiation, EF had improved. The other 4 with prior CHF had sub-normal EFs noted on echocardiograms done prior to initiation of CFZ (median EF of these 4 was 49%). Overall, baseline EF was available for 10 out of these 12 patients and median EF at baseline was 55% (range, 45 - 67%). Other risk factors predisposing to cardiac dysfunction were smoking in 1 patient and history of radiation to the chest in 2 (17%) patients. No patient had received cardiotoxic dose of anthracyclines prior to starting CFZ.
Myeloma therapies: All patients had received multiple lines of therapy for MM (median 4, range, 2-7). Ten (83%) patients had previous received bortezomib and 10 (83%) had received autologous stem cell transplant.
Cardiac events and CFZ use: Median duration of CFZ therapy received prior to documented systolic dysfunction was 4 months (range, 0.5 - 20 months), with 3 episodes happening with less than one month of total CFZ therapy. Five out of the 12 (42%) patients had previously discontinued CFZ for other reasons when the systolic dysfunction was noted. However they were included since CHF occurred shortly after treatment (median of 2 months, range, 0.5 - 3). The median drop in EF was 22% (range, 9 - 45%).
Recovery/ outcomes: Follow-up echocardiograms were available in 8 patients and all of these showed some improvement in EF (table 1). Median improvement in EF noted by the time of last follow up was 16% (range, 7-30%). Median best EF noted after the initial drop was 48% (range, 35 - 67%). Two patients never discontinued CFZ and EF improved spontaneously, to 67% and 64% respectively, after an initial drop. Another patient was rechallenged with CFZ due to progression of MM, 8 months after initial discontinuation, and did not have recurrence of systolic dysfunction. No patients succumbed to CHF. Six (50%) out of these 12 patients had died by the time of last follow-up. Cause of death was progressive MM in 4 (67%) and sepsis secondary to immunosuppression resulting from chemotherapy in 2 (33%).
Conclusions:
Our data, although with limited number of patients, suggests using caution when treating patients who have underlying cardiac risk factors with CFZ. Nevertheless, the cardiotoxicity was mostly reversible, with the minimal EF after recovery of 35%. In the setting of a life threatening malignancy, the impact and reversibility of cardiotoxicity needs to be understood so as not to prevent the utilization of otherwise effective therapy such as CFZ. Larger studies are needed to confirm these findings.
References:
1. Stewart et al. Carfilzomib, Lenalidomide and Dexamethasone for relapsed multiple myeloma. NEJM 2015;372:142-52.
Mikhael:Celgene: Research Funding; Abbvie: Research Funding; Sanofi: Research Funding; Onyx: Research Funding. Reeder:Novartis, Millennium, BMS and Celgene: Research Funding. Bergsagel:Amgen, BMS, Novartis, Incyte: Consultancy; Novartis: Research Funding. Fonseca:Millennium, a Takeda Company: Consultancy; Sanofi: Consultancy; AMGEN: Consultancy; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; Millennium, a Takeda Company: Consultancy; Novartis: Consultancy; AMGEN: Consultancy; Janssen: Consultancy; Janssen: Consultancy; BMS: Consultancy; Millennium, a Takeda Company: Consultancy; Bayer: Consultancy; Millennium, a Takeda Company: Consultancy; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Celgene: Consultancy; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; AMGEN: Consultancy; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Patent: Patents & Royalties: Prognostication of MM based on genetic categorization of FISH of the disease; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; Patent Pending: Patents & Royalties: The use of calcium isotopes as biomarkers for bone metabolisms; Celgene: Consultancy; BMS: Consultancy; Bayer: Consultancy; Novartis: Consultancy; Sanofi: Consultancy; AMGEN: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal