Abstract
Introduction: High throughput techniques, such as massively parallel sequencing, are becoming an attractive approach to characterize multiple myeloma (MM) genomic profiles. However, the clinical relevance and contribution to risk assessment of such approaches need to be established. The Multiple Myeloma Research Foundation (MMRF)CoMMpass trial (NCT01454297) has collected data from 1000 newly-diagnosed MM patients enrolled worldwide and observed through an expected follow-up of up to eight-years. Comprehensive analysis of somatic mutations detected in purified MM cells could reveal disease features with prognostic value, which may not have been detected using traditional approaches.
Materials and methods: We analyzed data from the interim analysis 8 cohort. CD138+ purified MM specimens from bone marrow aspirates and mononuclear cells from peripheral blood were collected at diagnosis. Whole exome libraries from both tumor and constitutional DNA samples were created. Somatic single nucleotide variants (SNV) were identified using three different variant callers (Seurat,Strelka andMutect), only nonsynonymous SNV calls made from at least two callers were included in the analysis.
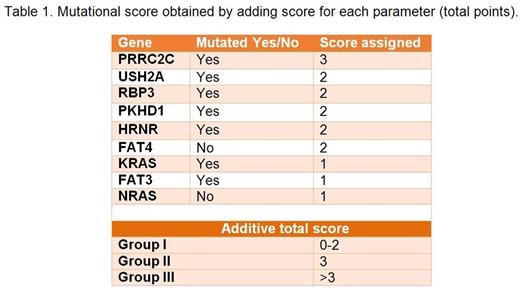
Patients were analyzed on an intention-to-treat basis. We evaluated the impact on progression free survival (PFS) of recurrently mutated genes (with at least a nonsynonymous SNV in more than 10 patients) in a Cox model adjusted for international staging system (ISS) and cytogenetic profile (high risk, standard risk and missing). An additive score related to mutated genes was calculated on the basis the level of each coefficient estimated using a Cox Model with backward selection based on theAkaikeInformation Criterion (AIC).
Results: 517 patients with baseline somatic mutation data were included in the analysis. Median age at diagnosis was 64 years (range 27-93). All patients received novel agents as first line treatment; 236 (45.6%) received autologous stem cell transplantation (ASCT). Each patient showed a median number of 55 nonsynonymous somatic SNV (range 8-1970) in a median number of 47 genes (range 5-1741). Excluding immunoglobulin genes, the most recurrent mutated genes were KRAS (25%), NRAS (19.5%), TTN (12.1%), MUC16 (9.1%) and DIS3 (9.1%). Based on the results of a multivariable Cox model corrected for ISS and cytogeneticprofile, we created a scoring system determined by the mutational status of 9 genes identified in a nonbiased manner (Table 1). Three groups were identified: group I (score 0-2, 17%); group II (score=3, 51%), and group III (score >3, 32%). After a median follow-up of 371 days, the 18-month PFS rate was 93% for group I, 85% for group II, and 67% for group III. In a Cox model adjusted for ISS and cytogeneticprofile, the hazard ratio was 2.34 (p=0.112) for group II versus group I, and 5.96 (p<0.001) for group III versus I. The prognostic trend of the score was confirmed in different patient subgroups including ASCT/no ASCT, standard/high risk cytogenetic profile, ISS I, II, or III. Of note, 23.3% of patients in group I had an ISS III and 30.4% of patients in group III had an ISS I.
Conclusion: The use of a prognostic model based on the mutational status of 9 recurrently mutated genes could improve risk assessment of newly-diagnosed MM patients. To our knowledge, this is the largest study correlating nonsynonymous somatic SNV with MM patients' outcome. Longer follow-up and validation in independent cohorts are needed to confirm our findings.
Larocca:Amgen, Celgene, BMS, Janssen-Cilag: Honoraria. Gay:Amgen: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees. Palumbo:Janssen Cilag: Honoraria; Takeda: Employment, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal