Abstract
INTRODUCTION: The benefits of ibrutinib (ibr) have been demonstrated by the phase 3 RESONATE and RESONATE-2 trials for patients (pts) with R/R and TN (≥ 65 years) CLL, respectively. In these studies, ibr showed significantly improved progression-free survival (PFS) and overall survival (OS) vs approved comparators (ofatumumab [ofa] in RESONATE and chlorambucil [clb] in RESONATE-2). However, a number of other treatment regimens are widely used in clinical practice for CLL based on individual patient and disease characteristics; it is currently unknown if ibr results in better survival outcomes when compared directly with each of these other treatments.
AIM: In the absence of a head-to-head comparison, we investigated the relative efficacy of ibr vs physician's choice (PC) in pts with R/R or TN CLL by comparing pt-level data from RESONATE and RESONATE-2 with data from pts in a real-world setting (the Lyon-Sud database). This database holds electronic medical records for 390 pts with CLL from the academic Lyon-Sud Hospital in France; nearly all patients were diagnosed between 1990 and 2014. This is the first analysis of ibr vs PC in a real-world setting for pts with TN CLL.
METHODS: Pt-level data from RESONATE (ibr, n = 195; ofa, n = 196) were compared with data on pts with CLL from Lyon-Sud who received 2nd(n = 107) or later-line treatment (3rd [n = 62], 4th [n = 43], and subsequent [n = 51] lines). Similarly, RESONATE-2 (ibr, n = 136; clb, n = 133) pt-level data were compared with those of pts aged ≥ 65 years who received 1st-line treatment (n = 131). In order to account for non-comparability due to lack of randomization, a multivariate Cox proportional hazards model was used to compare PFS and OS between treatments, including line of therapy, age, sex, disease stage (based on Binet/Rai), and deletion 17p or 11q mutations as covariates. For the definition of PFS, missing data for date of disease progression for pts in Lyon-Sud who initiated subsequent therapy were replaced by the conservative proxy of date of initiation of next treatment. Predicted PFS and OS curves for the Lyon-Sud cohort were derived from the multivariate model.
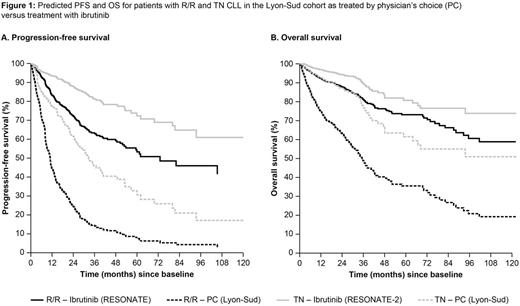
RESULTS: For R/R pts in Lyon-Sud, across all treatment lines the most frequent regimens used were rituximab (R) + chemotherapy (n = 46), bendamustine + R (BR; n = 28), fludarabine + cyclophosphamide + R (FCR) (n = 27), clb + R (n = 19), R monotherapy (n = 21), and R-CHOP-based (n = 19); the remaining percentages comprised various other therapies, each used in < 5% of pts. Median age at treatment initiation for Lyon-Sud and RESONATE was 69 and 67 years, respectively; median number of prior therapies was 2 and 3, and median follow-up was 30.0 and 21.9 months. More lines of prior therapy, older age, male sex, advanced disease stage (Binet C/Rai III/IV), and presence of deletion 17p/11q were independent risk factors for worse PFS and OS outcome. After adjustment for differences in baseline characteristics, PFS and OS hazard ratios (HRs) for ibr vs PC were 0.18 (95% confidence interval [CI], 0.13-0.26) and 0.28 (0.17-0.46), respectively. Adjusted HRs for ibr vs the most frequent treatments ranged from 0.09 (R monotherapy) to 0.38 (FCR) for PFS and 0.21 (R monotherapy) to 0.33 (FCR) for OS; all were statistically significant. For TN pts in Lyon-Sud, the most frequent treatment regimens used were FCR (n = 40), clb + R (n = 21), clb monotherapy (n = 17), R-(mini)CHOP (n = 11), and BR (n = 11). Median age at treatment initiation was 73 years for both Lyon-Sud and RESONATE-2; median follow-up was 36.9 and 28.1 months, respectively. Older age, male sex, and advanced disease stage were independent risk factors for worse PFS and OS outcome. After adjustment for baseline differences, the PFS and OS HRs for ibr vs PC were 0.25 (0.14-0.47) and 0.41 (0.17-0.99). Predicted PFS and OS curves for both R/R and TN patients are presented in Figure 1.
CONCLUSIONS: Investigation of survival outcomes suggests that ibr administered to pts in RESONATE and RESONATE-2 was more effective than PC used in a real-world cohort of French pts with R/R or TN CLL, suggesting a 4.8-fold improvement in PFS and a 3.4-fold improvement in OS in R/R pts and 4-fold and 2.4-fold improvements in TN pts, respectively. These results further support the growing evidence that ibr significantly improves both PFS and OS vs commonly used regimens in the R/R and TN settings, and could have important implications for improving treatment of CLL in clinical practice.
Salles:Janssen: Consultancy, Honoraria; Gilead: Honoraria, Research Funding; Mundipharma: Honoraria; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding. Sarkozy:Sandoz: Research Funding; Janssen Research & Development: Honoraria; Gilead: Honoraria; Takeda: Research Funding. Ghesquieres:Mundipharma: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche France: Research Funding. Diels:Johnson & Johnson: Employment, Equity Ownership. Besson:Janssen: Employment. MacDougall:Janssen Research & Development: Research Funding; IMS Health: Employment. Hermans:Janssen Research & Development: Research Funding; IMS Health: Employment. Healy:Janssen Research & Development: Employment. Garside:Janssen Research & Development: Employment. Iraqi:Janssen Research & Development: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal