Abstract
Introduction
In transfusion dependent thalassemias (TDT), plasma risk factors predicting myocardial hemosiderosis (MH) are needed. High labile plasma iron (LPI) and non-transferrin bound iron (NTBI, in its various species) may increase MH risk, so could be blood biomarkers, but the clinical utility seems unclear (Garbowski et al. 2015 Blood). This is partly due to the characteristics of the LPI assay and the two NTBI assays: the NTA-assay and the bead assay. Firstly, the former two assays are inhibited in the presence of iron free ligands (Aydinok et al. 2012 Haematologica; Walter et al. 2008 Haematologica), the NTBI bead assay less so (Ma et al. 2014 Biochem J; Garbowski et al. 2016 Transl Res). Secondly, the assays capture iron-chelate complexes to a variable extent: the NTA assay detects both deferasirox (DFX2Fe) and deferiprone complexes (DFP3Fe), the NTBI bead assay only detects the latter (Garbowski et al. 2016 Transl Res) - meaning that clinical utility is confounded without with-holding chelator (impractical) (Aydinok et al. 2012 Haematologica). However, sampling on the day of chelation could be useful: iron-chelator complexes could reflect chelatable body iron (Aydinok et al. 2012 Haematologica), another potential MH risk biomarker. We therefore determined whether using the assays on the day of chelation, capturing NTBI and iron-chelator complexes together could identify patients with the greatest MH risk.
Patients and Methods
29 TDT patients included balanced proportions with and without MH (measured by CMR). Blood samples were taken on the day of CMR, without withholding chelation. All chelation modalities were accepted: DFX (n=13), DFO (n=7), DFP (n=1), DFO+DFP (n=4), DFO+DFX (n=1), DFX+DFP (n=1) or no chelation (n=2). NTBI was determined by both the NTA and bead-NTBI method (Ma et al. 2014 Biochem J). LPI was measured as described (Esposito et al. 2003 Blood). Regression of MH against NTBI was analysed in patients receiving same day chelation (n=16) or last chelation prior to this (n=13).
Results
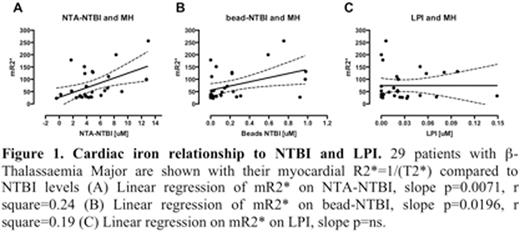
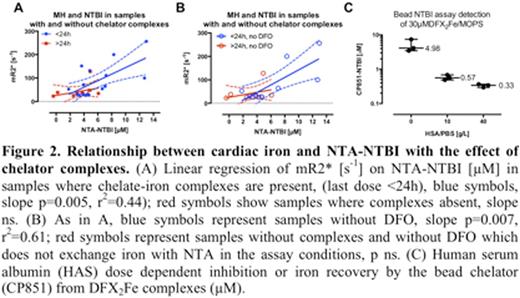
14/29 had MH (T2* <=20ms, range 3.9-20ms. NTA-NTBI was higher in MH(+) than MH(-) patients (6.1 vs 3.8 microM, p=0.04). R2* (1/T2*) correlated with NTA-NTBI (Figure 1A), but weakly with the bead-NTBI (Figure 1B). By contrast no relationship between LPI and MH was found (Figure 1C). NTA-NTBI values were markedly higher than the bead-NTBI (4.8 vs 0.2 microM, p<0.0001), particularly where samples were taken <24h from last chelator dose (5.8 vs 0.3 microM, p<0.0001), suggesting that iron complexes of chelators detected by the NTA method might be driving the relationship of NTBI with MH. There was a highly significant (p=0.005, r2=0.44) relationship between MH and NTBI in blood samples taken within 24h of chelator dosing (Figure 2A) but not where 24h had elapsed (slope p=0.33). As neither the bead nor the NTA methods detect ferrioxamine (Ma et al. 2014 Biochem J), we then excluded DFO monotherapy samples. This strengthened the relationship of NTA-NTBI to R2* in the remaining samples (r2=0.6, p=0.0003, Figure 2B). The LPI results (range 0-0.15 microM) were all within the normal reference range, consistent with LPI lowering effects of iron free chelators outweighing increasing effects of chelate complexes (Zaninelli et al. 2009 BJH) (Figure 1C). The bead-assay detection of iron from DFX2Fe is abolished in vivo by 40g/L of albumin (Figure 2C), explaining the low values of the bead-NTBI assay.
Discussion
These findings suggest a relationship between MH and chelatable iron pools. These are detected as plasma iron complexes by the NTA-NTBI method, which has not been previously reported. This is only clear where chelation is given on the day of blood sampling and reflects iron chelated from both plasma and hepatic iron turnover. This holds for patients receiving DFP and DFX but not DFO, where iron complexes are not visible to the NTA assay. This relationship is also not seen with the bead or LPI assays because iron complexes are less uniformly detected. These findings implicate the magnitude of chelatable iron pools to MH risk.
Conclusion
MH in TDT correlates with plasma levels of chelate complexes of DFP and DFX, detected by the NTA-NTBI assay, measured on the day of chelator administration suggesting that blood iron-chelator complexes could be a biomarker for myocardial iron hemosiderosis.
Porter:Novartis: Consultancy, Honoraria, Research Funding; Celegene: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria; Bluebird Bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal