Abstract
The complex interplay between bone marrow-derived mesenchymal stromal cells (BM-MSC) and neoplastic hematopoietic cells is involved in the progression of myeloproliferative neoplastic (MPN) diseases. Extracellular vesicles (EV) have emerged as a complex cell-to-cell communication system within the neoplastic microenvironment. EV are able to reprogram recipient cells by transferring proteins, mRNA and microRNA from their cell of origin. We aimed to analyze the microRNA content of EV obtained from MPN BM-MSC, as well as the changes induced when these EV are incorporated into hematopoietic progenitor cells (HPC).
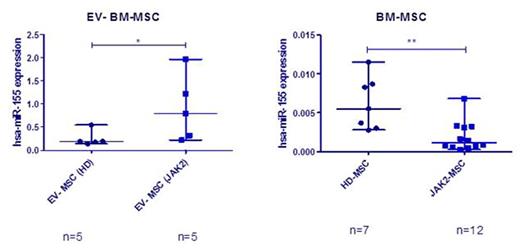
EV were isolated from BM-MSC of MPN patients (n=22) and healthy donors (HD) (n=19) by ultracentrifugation. Characterization of EV by transmission electron microscopy (TEM), immunoblot, multiparametric flow cytometry (MFC) and NanoSight analysis revealed vesicles with a typical bilayer-membrane characteristic morphology with a size inferior to 500 nm, which were positive for various EV markers as CD63 and CD81, and for MSC markers as CD73, CD90 and CD44 (Figure 1). MicroRNA profiling by 384-well microfluidic cards (TaqMan® MicroRNA Array A) showed an overall increase in the microRNA expression in the MPN-MSC-derived EV, when compared to EV from donor MSC. Using RT-PCR, we observed that miR-155 was selectively enriched in EV released by MPN-MSC. An overexpression of this microRNA was observed in EV (p=0.032), while a downregulation was observed in BM-MSC (p=0.0078) (Figure 2).
EV incorporation was demonstrated by fluorescence microscopy and MFC, where HPC (CD34+ cells obtained by immunomagnetic selection) were co-cultured with Vybrant Dil-labeled EV. For functional studies apoptosis and clonogenic assays (CFU-GM) were performed. We observed an increase in CD34+ cell viability after incorporating EV from BM-MSC (HD and MPN). Moreover, an increase (p=0.04) in miR-155 expression was observed when HD HPC incorporated EV from MPN-MSC. When neoplastic CD34+ cells incorporated the EV derived from MPN-MSC an increase of CFU-GM number was also observed.
We suggest that EV released from MPN-MSC represent a mechanism of intercellular communication between malignant stromal and hematopoietic cells, through the transfer of genetic information that may be relevant in the pathophysiology of these diseases.
Funding: GRS 1034/A/14 (C. Sanidad, JCYL) and FCT (SFRH/BD/86451/2012)
EV characterization by TEM (A), Immunobloting - CD63 (B) and MFC (C). Scale bar: 200 and 500 nm.
EV characterization by TEM (A), Immunobloting - CD63 (B) and MFC (C). Scale bar: 200 and 500 nm.
Expression of miR-155. RT-PCR from EV released from HD and MPN-MSC (A), and the expression of miR-155 in BM-MSC (B).
Expression of miR-155. RT-PCR from EV released from HD and MPN-MSC (A), and the expression of miR-155 in BM-MSC (B).
Sánchez-Guijo:Bristol-Myers-Squib: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria. Del Cañizo:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jansen-Cilag: Membership on an entity's Board of Directors or advisory committees, Research Funding; Arry: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal