Abstract
Background: Although it may vary between different registries, the median age of chronic myeloid leukemia (CML) at diagnosis is between 50-60 years, and approximately 40% of the patients (pts) are diagnosed after age 60 [Hoffmann et al. Leukemia. 2015;29(6):1336-43]. Tyrosinekinase inhibitors (TKIs) have revolutionized the treatment of CML and currently pts with CML may expect to live close to normal lifespan. So the number of elderly CML pts with various potentialcomorbidities started to increase, which then brings out the issues regarding TKI toxicities, medication adherence and responses to TKI treatment.
Aim: The aim of this study was to evaluate the efficacy and safety ofimatinib treatment in the elderly population (pts equal to or older than 60 years) with CML and to compare these data with younger pts (pts < 60 years).
Patients and Methods: Pts diagnosed and followed in our clinic with CML were enrolled in the study. Patient demographics, dose and duration ofimatinib therapy, disease risk scores, cytogenetic and molecular responses,comorbidities, adverse events (AEs), follow-up durations and outcomes were evaluated from files of the pts, retrospectively. TheCharlsoncomorbidity index (CCI) of each patient was calculated as stated before [Breccia et al.Haematologica. 2011;96(10):1457-61].
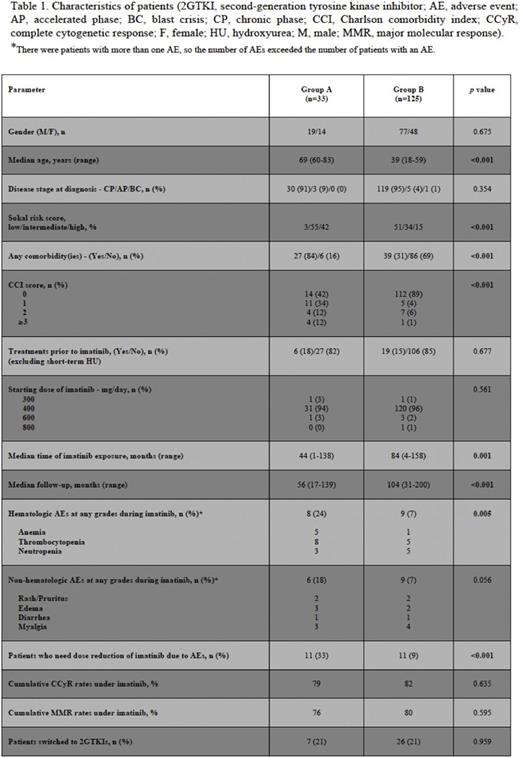
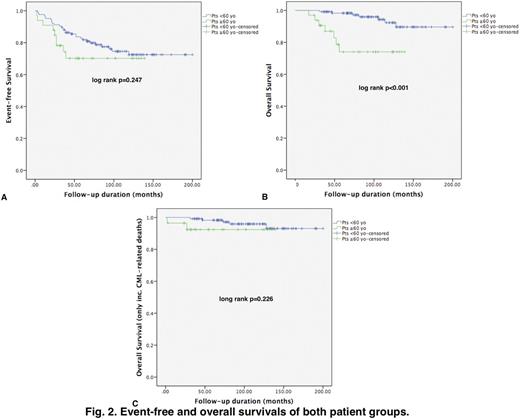
Results: The patient cohort was consisted of 158 pts with a median age of 44 years (range, 18 - 83 years). Group A consisted of thirty-three pts who were equal to or older than 60 years (Fig. 1), and there were 125 pts (Group B) who were < 60 years of age (Table 1). The two groups were balanced regarding gender, disease stage, treatments prior to TKI therapy, and the starting dose ofimatinib. There were more pts with intermediate and highSokal risk scores in Group A than that of Group B (p<0.001). Pts in Group A had significantly morecomorbidities (p<0.001) and higher CCI scores (p<0.001) than pts who were < 60 years of age (Table 1). Median time ofimatinib exposure (p=0.001) and follow-up durations (p<0.001) were significantly longer in Group B than those of Group A. There were significantly more hematologicAEs among pts in Group A than pts in Group B (24% vs. 7%, p=0.005). Although not statistically significant, non-hematologicAEs were more common in GroupA (18% vs. 7%, p=0.056), and the rates ofimatinib dose reduction due toAEs were significantly higher in Group A than Group B (33% vs. 9%, p<0.001). Cumulative complete cytogenetic (CCyR) and major molecular (MMR) response rates and the percentage of pts who switched to second-generationTKIs (2GTKIs) were similar in both groups (Table 1). Event-free survival (EFS) rates were comparable (Fig. 2A), however overall survival (OS) rate was significantly higher in Group B (Fig. 2B) (p<0.001). There were 7 pts in both groups who died during the follow-up, but five and 2 pts died due to non-CML related causes in Groups A and B, respectively. OS rates were similar in both groups when non-CML related deaths were excluded (Fig. 2C).
Discussion: TKI therapy is relatively safe in elderly pts with CML, and cytogenetic and molecular responses are comparable with that observed in younger pts. In our institute, where pts are followed in a dedicated outpatient CML clinic, cytogenetic and molecular responses were similar in both elderly and younger pts leading to similar percentages of pts who were switched to 2GTKI therapy in both groups. Comorbidities can be problematic in elderly pts in whom CML and TKI relatedAEs are more common. However proper management of theseAEs including careful follow-up and timely TKI dose reductions may lead to successful outcomes. Inferior OS rates were observed in elderly pts with CML, but OS rates were similar in both groups when non-CML related deaths were excluded.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal