Abstract
Background:
The etiology of chronic myeloid leukemia (CML) is essentially unknown with high doses of ionizing radiation being the only well established risk factor. We have recently published two large population-based studies showing an increased prevalence of other malignancies prior, as well as subsequent to a diagnosis of CML (Gunnarsson et al, Br J Haematol. 2015 Jun; 169(5): 683-8 and Gunnarsson et al, Leukemia. 2016 Jul; 30(7): 1562-7). One may therefore speculate that CML patients may have an increased congenital or acquired susceptibility to develop cancer. In the former case, one would expect an increased prevalence of malignancies among first-degree relatives (FDR) to CML patients.
In a previous report based on the Swedish Cancer Registry, no increased aggregation of malignancies was detected among family members to CML patients diagnosed between 1958 and 2004 (Bjorkholm et al, Blood. 2013 Jul 18; 122(3): 460-1). However, a more strict definition of CML (requiring e.g. thepresence of a Philadelphia chromosome or the BCR/ABL fusion gene) was introduced with the updated WHO classification in 2002, making subsequently diagnosed CML cohorts more homogenous.
Materials and methods:
Aiming to establish the prevalence of malignancies among FDR of a large and well-defined contemporary CML cohort in Sweden compared to carefully matched controls, we have used four large Swedish population based registers.
To identify Swedish patients with CML diagnosed between 2002 and 2013, we used the Swedish CML Register to which all CML patients diagnosed January 1st 2002 and later are reported. FDR were identified by use of the Swedish Multi-Generation Register, which comprises information about parent-sibling-offspring relationships of persons that has been registered in Sweden at some time since 1961 and born later than 1932.
By linking this cohort to the Swedish Cancer Register, we retrieved information about malignancies for each FDR.
Each CML patient was matched with five, age-, gender- and county of residence-matched controls, selected from the Swedish Total Population Register. All controls had to be alive and free of CML at the time of CML diagnosis for the matching CML patient.
To calculate odds ratio (OR) and 95% confidence intervals (CI), conditional logistic regression were used.
Results:
In the Swedish CML register, 984 patients were identified. Among them 184 patients were excluded due to a birth year prior to 1932. Among the 800 remaining CML patients, 4 287 FDR were identified and included in the analysis (parents: 1 346, siblings: 1 497 and children: 1 444). Correspondingly, 20 930 matched controls were included in the analysis.
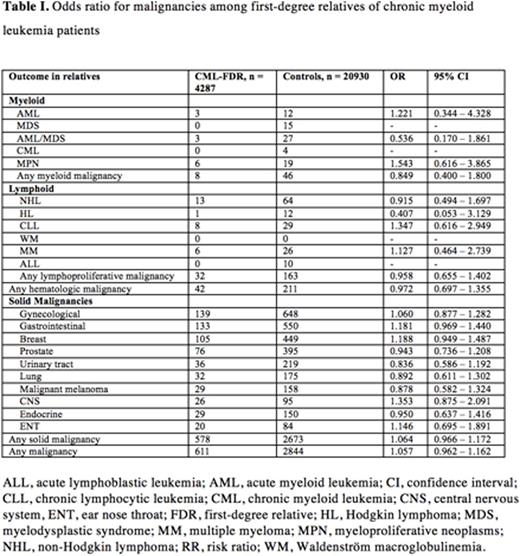
In total, 611 malignancies were identified among the FDR of CML patients compared to 2844 in the control group yielding an OR of 1.057 (95% CI 0.962 - 1.162).
Neither hematological malignancies nor solid tumors were increased in the CML-FDR group (Table 1). Notably, none of the FDRs in the CML-FDR group had a CML diagnosis.
Conclusions:
Using data from four large Swedish population based registers and based on the fate of more than 4 000 FDR of 800 CML patients diagnosed in the modern era of cytogenetics, as well as closely matched CML-free controls, we show that there is no familial aggregation of malignancies in FDRs of patients with CML. These results suggest that a hereditary predisposition to develop cancer is unlikely to be a part of the pathogenesis of CML.
Höglund:Akinion Pharmaceuticals: Consultancy; Janssen-Cilag: Honoraria. Lambe:AstraZeneca: Other: Stock Ownership ; Pfizer: Other: Stock Ownership . Richter:Pfizer: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Ariad: Honoraria, Research Funding. Själander:ARIAD: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal