Abstract
Introduction: Immunotherapy with rituximab alone or in combination with sequential chemotherapy such as CHOP, in addition to a reduction in immunosuppression (IST), has been shown to be effective in achieving long-term, disease-free survival in patients with B-cell PTLD. We have recently observed an increased incidence of HGBL in patients receiving IST following solid-organ transplant. Intensive induction regimens (ex: DA-R-EPOCH) in non-transplant HGBL has been associated with improved complete responses. Intensive regimens have not been previously evaluated in patients with PTLD. The aim of this study is to compare the tolerability of DA-R-EPOCH to R-CHOP in post-transplant patients with HGBL.
Methods: Patients treated with either DA-R-EPOCH or R-CHOP were included in this study following IRB approval. Eligible patients were ≥18 years, had biopsy-confirmed B-cell PTLD, and were treated with at least one cycle of DA-R-EPOCH or R-CHOP. The primary outcome was progression-free survival (PFS); secondary outcomes were overall survival (OS), toxicities, and hospitalizations due to treatment-related toxicities. Statistical analysis was performed using SPSS.22 software
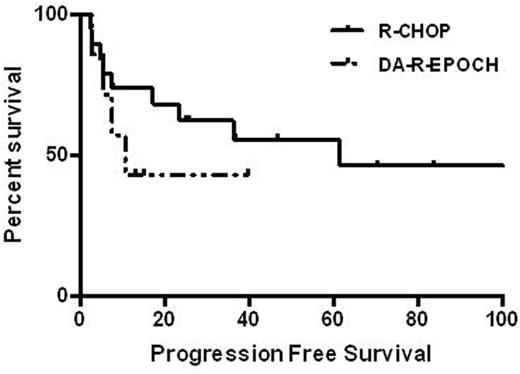
Results: Sixty-three patients had biopsy-confirmed PTLD. Of these, 26 met inclusion criteria. Among these 26 patients, 19 (73.1%) were men; median age was 57 years (18-75 years); and transplants included 3 (11.5%) lung, 4 (15.4%) heart, 9 (34.6%) kidney, 8 (30.8%) liver, 2 (7.7%) pancreas, and 1 (3.8%) stem cell. All patients were receiving IST at the time of diagnosis of PTLD. Pathology reports found that 24 (92.3%) had diffuse large B-cell lymphoma (DLBCL)-like PTLD, and 11 (57.9%) had EBV-positive disease. HGBL was observed in 10 (38.5%) patients. Seven patients received DA-R-EPOCH, and 19 received R-CHOP. Baseline characteristics were similar between treatment groups. There was a significantly higher number of patients with HGBL in the DA-R-EPOCH arm compared with the R-CHOP arm (100% [7/7] vs. 15.8% [3/19]; 95% CI, -0.01-1.00; p=0.001). The median number of cycles administered was not significantly different between the groups (4.6 cycles vs. 5 cycles; 95% CI, 4.33-6.07; p=0.645). Dose intensification occurred in 8 of 32 cycles for patients who received DA-R-EPOCH. The median follow-up time for patients treated with DA-R-EPOCH was shorter (10 months) than for patients treated with R-CHOP (29 months). PFS was not found to be significantly different between the DA-R-EPOCH and R-CHOP arms (10.4 months vs. 61.4 months; 95% CI, 1.80-18.99; p=0.31). Patients with EBV-positive disease had inferior PFS compared with EBV-negative disease (7.37 months vs. NR; 95% CI, 1.02-10.2; p=0.046). In addition, OS, neutropenia, thrombocytopenia, hospitalizations, and hospitalizations due to febrile neutropenia were not significantly different between groups, though trends toward higher rates of grade 3 or 4 neutropenia, thrombocytopenia, and hospitalizations was observed in the DA-R-EPOCH group.
Conclusion: To our knowledge, this is the first study evaluating the role of intensive induction therapy in patients with HGBL with MYC and BCL2 rearrangements observed in solid organ transplant recipients. In patients with PTLD, DA-R-EPOCH is a well-tolerated regimen with concurrent taper in IST. However, this strategy may not overcome the poor prognosis of HGBL. Dose adjustments beyond level 2 were limited by cytopenias.
Reddy:KITE: Membership on an entity's Board of Directors or advisory committees; celgene: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees; INFINITY: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal