Abstract
Introduction: Progressive disease on ibrutinib is an emerging area of an unmet need. The key mechanism of ibrutinib resistance is driven by the growth of subclones with BTK and/or PLCG2 mutations under selective pressure from therapy [Woyach JA et al, N Engl J Med 2014; Burger et al, Nat Commun 2016]. It is unclear, however, how to effectively discriminate patients who are at risk of progression during ibrutinib. Established prognostic factors in CLL were developed in the context of chemoimmunnotherapy, and only limited data are available on predictive values of complex karyotype [Thompson et al, Cancer 2015] and deletion 17p [Byrd et al, Blood 2015] in patients receiving ibrutinib. To determine predictive values of conventional prognostic factors in CLL, we performed multivariate analyses of factors relevant to progression-free survival (PFS) on ibrutinib.
Methods and Patients: 84 evaluable CLL patients received single-agent ibrutinib 420mg once daily under a phase II investigator-initiated trial (NCT01500733). 3-year safety and efficacy results from this trial were previously reported [Farooqui et al, 2015 ASH Annual Meeting Abstract 2937]. Briefly, eligible patients had either TP53 aberration (deletion 17p by FISH or TP53 mutation by targeted sequencing) or were 65 years or older. The study included both treatment-naive and relapsed/refractory patients. Metaphase karyotype was not routinely performed. The study was approved by the institutional review board, and conducted in accordance with the Declaration of Helsinki. Cox regression models were used to identify independent predictors of progression. Probability of PFS was estimated by the Kaplan-Meier method. Log-rank test was used to compare PFS probabilities between subgroups. Statistical analyses were performed using R statistical software version 3.2.3.
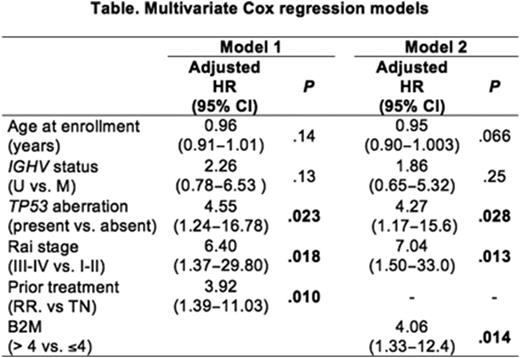
Result: At the median follow up of 34.3 months, 19 (22.6%) of 84 evaluable patients progressed or died on study; 15 (17.9%) patients progressed and 4 (4.8%) additional patients died due to reasons unrelated to progression. Independent factors of PFS identified from univariate analysis were; TP53 aberration, prior treatment, advanced Rai stage (III/IV) and high beta-2 microgloblin (B2M) (>4mg/L or =<4mg/L) (P<0.05 for all tests). Patients with unmutated IGHV had a trend towards inferior outcome compared to mutated IGHV, but the difference between two groups did not reach statistical significance (P=0.088). Six variables were considered for multivariate models; age (continuous variable), IGHV mutation, TP53 aberration, Rai stage, prior treatment and B2M. B2M was significantly correlated with previously treated disease (median B2M 5.2 mg/L in relapsed/refractory CLL vs. 3.3 mg/L in previously untreated CLL, P=0.0006). To avoid multicollinearity, we performed two multivariate Cox regression models separately including B2M (model 1) or prior treatment status (model 2) (Table). Two models showed similar predictive values for PFS (c-statistic: 0.80 for model 1, 0.79 for model 2). Multivariate analysis identified the presence of TP53 aberration, advanced Rai stage, and high B2M or prior treatment were independently associated with PFS. PFS of patients who had all three factors on either model 1 or model 2 were significantly inferior than those who had two or less factors (P=5.49x10-5 for model1, P=5.95x10-6 for model 2; Figure). The median PFS was 38.8 moths for those who were high-risk by model 1 and 38.4 months for those who were high risk by model 2, and was significantly shorter than non-high-risk patients (median PFS: not reached in both models).
Conclusion: This is the first integrated risk scoring system developed from patients receiving ibrutinib If confirmed in independent cohorts this model could enable early risk stratification for treatment trials with combination regimens or with alternative targeted agents. Conversely, the absence of adverse prognostic factors can be used to identify a subgroup who may benefit from single-agent ibrutinib and can potentially discontinue therapy if deep responses are achieved after long-term ibrutinib.
Farooqui:Merck: Employment. Wiestner:Pharmacyclics: Research Funding; Acerta Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal