Abstract
Introduction: The PETAL trial is a multicenter randomized controlled study for patients with aggressive lymphomas of diverse histologies (EudraCT 2006-001641-33, NCT00554164). In the study population as a whole interim PET (iPET) reliably predicted time to treatment failure (TTTF) and overall survival (OS). Interim PET-based treatment changes, however, had no impact on outcome (ASH 2014, abstract 391). Here we report the exploratory analysis for peripheral T cell lymphomas (PTCL).
Methods: Pts. aged 18 to 80 yrs. with newly diagnosed aggressive lymphomas and a positive baseline PET received 2 cycles of rituximab (R), cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) followed by iPET. R was omitted in pts. with CD20-negative lymphomas. The conditions of iPET were strictly defined: 3-week interval between the 2nd (R-)CHOP cycle and iPET to avoid inflammatory reactions (Eur J Nucl Med Mol Imaging 30:682, 2003), no G-CSF after the 2nd cycle to avoid altered glucose biodistribution (J Nucl Med 47:950, 2006), standardized uptake value (SUV)-based PET interpretation to improve reproducibility (favorable iPET response: reduction of maximum SUV by > 66 % compared to baseline; J Nucl Med 48:1626, 2007). PTCL pts. with CD20-negative lymphomas and a favorable iPET uniformly received 4 additional cycles of CHOP (part A of the trial). Pts. with an unfavorable iPET were randomized to continue CHOP for 6 additional cycles or receive 6 blocks of a more complex methotrexate-, cytarabine- and etoposide-based regimen originally designed for Burkitt lymphoma (Blood 124: 3870, 2014; part B). Sample size of the entire study population was based on the empirically derived assumption that treatment failure after 2 yrs. (progression, relapse, treatment discontinuation due to toxicity, start of alternative therapy, death of any cause) could be improved from 30 % to 45 % in part B (alpha=0.05, power=0.8). Secondary endpoints included OS and toxicity.
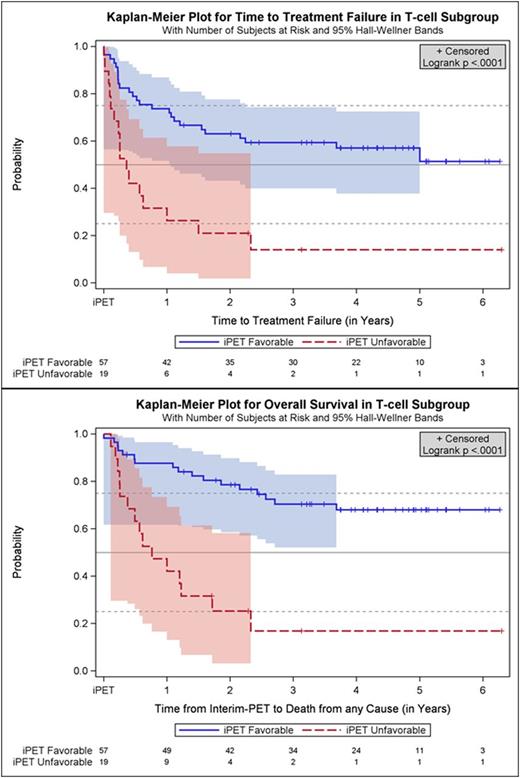
Results: Fifty-seven oncological centers and 23 nuclear medicine institutions participated in the trial. Between 2007 and 2012 1072 pts. were registered, and 862 (80.4 %) had a positive baseline PET, received 2 cycles (R-)CHOP, underwent iPET and were allocated to one of the post-iPET treatment arms detailed above. Reference pathology was available in 98 %, and median follow-up is 52 months. All in all, there were 76 pts. (8.8 % of all treated pts.) with T-cell lymphomas of whom 21 had ALK+ anaplastic large cell lymphoma (ALCL), 13 ALK- ALCL, 18 angioimmunoblastic T-cell lymphoma (AITL) and 20 PTCL not otherwise specified (NOS). Interim PET was favorable in 57 pts. (75 %) and unfavorable in 19 pts. with T-cell lymphomas (25 %). It was highly predictive of outcome, TTTF and OS being significantly higher in part A than B (2-year probability for TTTF: 63 % vs. 21 %; univariate hazard ratio (HR) for B 3.4, 95 % confidence interval (CI) 1.8 - 6.4, p<0.0001; OS: 79 % vs. 25 %; univariate hazard ratio (HR) for B 5.0, 95 % CI 2.4 - 10.3, p<0.0001; Figure). Interestingly, the proportion of T-cell lymphoma pts. with an unfavorable iPET response was more than twice as high as the corresponding proportion of B-cell lymphoma pts., and the difference in survival between pts. with a favorable vs. unfavorable iPET response was more pronounced in T-cell lymphomas than in B-cell lymphomas.
TTTF (2-year probability: 81 % vs. 46 % vs. 49 % vs. 35 %; p=0.0110) and OS (90 % vs. 69 % vs. 52 % vs. 50 %; p=0.0026) were better in ALK+ ALCL than in ALK- ALCL, AITL or PTCL NOS. In pts. with an unfavorable iPET response, a switch from CHOP to the alternative regimen failed to improve TTTF or OS. The latter was associated with more frequent grade 3/4 neutropenia (40 % vs. 0 % vs. 11 %, p=0.0279), thrombocytopenia (70 % vs. 33 % vs. 23 %; p=0.0106), infection (60 % vs. 44 % vs. 18 %, p=0.0057) and mucositis (40 % vs. 33 % vs. 4 %, p=0.0025) as compared to 6 or 4 post-iPET cycles of CHOP, respectively, but treatment-related mortality was similar in all treatment arms (2 vs. 1 vs. 2 deaths).
Conclusion: In this large multicenter trial iPET proved highly predictive of outcome in PTCL. A favorable iPET was found in 75 % of pts., and this was associated with long-term survival in about 70 %. In pts. with an unfavorable iPET response, outcome was dismal and could not be improved by switching to a more aggressive regimen. Novel strategies are required for PTCL pts. failing to respond to the first 2 cycles of CHOP.
Hüttmann:Gilead, Amgen: Other: Travel cost; Bristol-Myers Squibb, Takeda, Celgene, Roche: Honoraria. Giagounidis:Celgene Corporation: Consultancy. Klapper:Roche, Novartis, Amgen, Takeda: Research Funding. Duehrsen:Roche: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Alexion Pharmaceuticals: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal