Abstract
Background: Idelalisib, a selective oral inhibitor of PI3Kd, is approved for the treatment of chronic lymphocytic leukemia (CLL) in combination with rituximab and as monotherapy for patients with follicular lymphoma who have received at least 2 prior therapies. Despite remarkable clinical efficacy, complete responses are rare, highlighting the need to identify more effective therapies, including combinations of novel agents. GS-4059 (ONO-4059) is an investigational next generation Bruton's tyrosine kinase (BTK) inhibitor with improved selectivity compared with ibrutinib. We report here the results of the combination of a PI3Kd inhibitor and GS-4059 in a diffuse large B-cell lymphoma (DLBCL) xenograft model, demonstrating supportive data for our ongoing combination trial in B-cell malignancies (NCT02457598). Additionally, we investigated preclinical orthogonal combination approaches for DLBCL.
Methods: Growth inhibition was assessed using CellTiter-Glo Assay after 96 h incubation with idelalisib and GS-4059. CB17-SCID mice were irradiated, implanted subcutaneously with TMD8, and treated BID PO with the PI3Kd inhibitor GS-649443, GS-4059, or coformulated combination when tumors reached 200 mm3. Lysates from tumors or cell cultures were analyzed by Simple Western (Protein Simple). Synergy for antiproliferative effects was assessed using Chalice software (Horizon Discovery, Inc., Lehar et al., Nature Biotech, 2009).
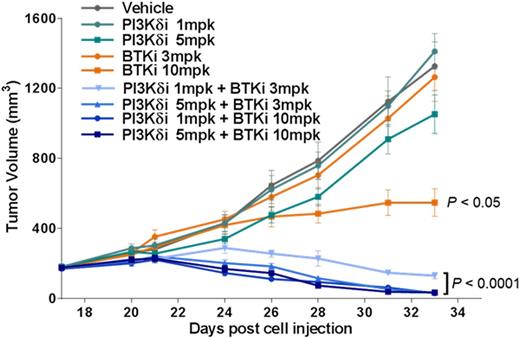
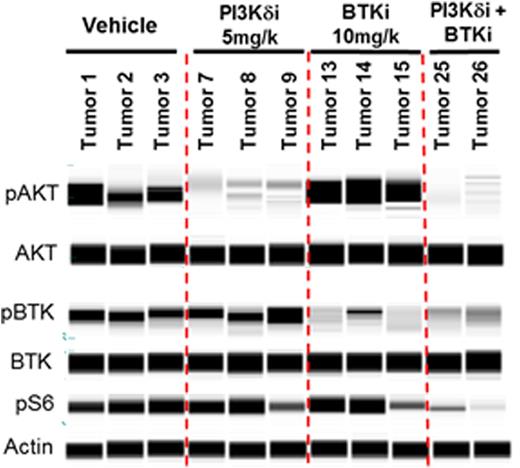
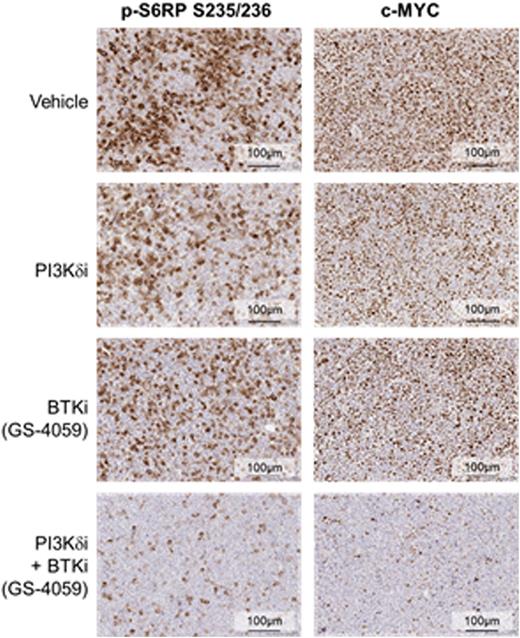
Results: Idelalisib and GS-4059 potently inhibited the ABC subtype DLBCL cell line TMD8, which is a B-cell receptor (BCR)-dependent line that exhibits chronic activated B-cell signaling due to mutations in CD79A/CD79B and MYD88 (Kim Y. et al., Hum Pathol, 2014). When a clinically relevant single concentration of idelalisib or GS-4059 was added in combination to a dose responsive effect of the other, a shift in EC50 on cell viability was seen. GS-4059 (50 nM) shifted the EC50 of idelalisib from 141 nM to 5 nM, a 28-fold shift. Idelalisib (1 µM) shifted the EC50 of GS-4059 from 27 nM to 2 nM, a 14-fold shift. Evaluation of downstream signaling pathways implicated in malignant B-cell survival and proliferation showed enhanced inhibition of pAkt S437, pBTK Y223, pErk1/2 T202/Y204, and MYC with a combination of idelalisib and GS-4059, more than either single agent alone. When TMD8 xenografts were treated with a PI3Kd tool compound, GS-649443, GS-4059 or a combination of the 2 inhibitors, a statistically significant decrease in tumor volume was seen as well as tumor regression, when compared with single agent effects (Figure 1A). Evaluation of TMD8 tumor lysates showed strong suppression of pAkt S437, pBTK Y223, pS6RP S235/236, and MYC in tumors treated with both GS-649443 and GS-4059 (Figure 1B). pS6RP S235/236 and MYC, in formalin-fixed paraffin-embedded (FFPE) TMD8 tumors, were profoundly inhibited in tumors treated with combination therapy compared to the monotherapies (Figure 1C). Since the combination of a PI3Kd inhibitor and GS-4059 led to TMD8 tumor regression, an effect correlated to strong down-modulation of MYC, the combination of idelalisib with a bromodomain and extra-terminal (BET) family inhibitor was explored as a potential new orthogonal combination approach for DLBCL. A panel of DLBCL cell lines was evaluated for inhibition of cell viability by idelalisib in combination with GS-5829, a BET inhibitor currently being evaluated in a phase 1 clinical trial. At clinically relevant concentrations, the combination of idelalisib and GS-5829 showed synergistic effects on cell viability in 2 of 6 ABC subtype, 4 of 5 GCB subtype, and 2 of 2 double-hit DLBCL cell. As compared with combination with other agents that inhibit the BCR pathway (GS-4059) or the Bcl-2 pathway (ABT-199), the broadest activity across cell lines was seen with the combination of idelalisib and GS-5829.
Conclusion: Idelalisib and GS-4059 demonstrated synergistic inhibition of the TMD8 xenograft with concomitant inhibition of MYC. Screening of other targeted agent combinations in a panel of DLBCL lines revealed broad preclinical activity for the BET inhibitor GS-5829 in combination with idelalisib. This represents a potential orthogonal approach for a new therapeutic strategy for the treatment of B-cell malignancies.
Meadows:Gilead Sciences: Employment. Yahiaoui:Gilead Sciences: Employment. Sorensen:Gilead Sciences: Employment. Cui:Gilead Sciences: Employment. Brockett:Gilead Sciences: Employment. Keegan:Gilead Sciences: Employment. Tannheimer:Gilead Sciences: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal