Abstract
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of lymphomas in which only ~25% of patients experience long-term survival with CHOP chemotherapy. Recently several drugs have been approved for this entity including pralatrexate (P), romidepsin (R), and belinostat which have response rates ranging from 26%-29% as single agents. Based on our demonstration of synergy of P+R in preclinical models of TCL, we initiated a study on the safety and efficacy of P+R in a phase I-II study for relapsed or refractory lymphomas (NCT01947140) and sought to evaluate biological mechanisms of synergy.
A 3+3 dose-escalation study started at P 10mg/m2 and R 12mg/m2 with escalation to P 25 mg/m2 and R 14 mg/m2. Patients were treated on 1 of 3 dosing schedules (weekly x 3 Q28D; weekly x 2 Q21D and QOW Q28D). The primary objective was to determine MTD and DLT; the secondary objective included describing ORR (CR+PR). Patients were required to have relapsed lymphoma of any subtype, ECOG PS ≤2, and adequate organ and marrow function. There was no upper limit to the number of prior therapies or transplantation.
Twenty-six patients were enrolled and were evaluable for toxicity. Median age was 52 yrs (23-73) and 58% were male. The median number of prior therapies was 3 (range 1-16). Histologies included HL (N=3), B-cell (N=10 of which FL=4) and T-cell (N=13). The median number of cycles completed was 4 (range 1-12). There were 3 DLTs in cohort 4 (P 20mg/m2 & R 12mg/m2given weekly x 2 Q21D) consisting of 2 Grade 3 oral mucositis and 1 Grade 4 sepsis. The QOW Q28D schedule had no mucositis at all dose levels. Patients dosed at the MTD (P 25 mg/m2 & R 12mg/m2 QOW) did not experience any toxicities. The grade 3/4 toxicities reported in >5% of patients were: neutropenia (31%), thrombocytopenia (31%), anemia (23%), oral mucositis (15%), hyponatremia (8%), pneumonia (8%) and sepsis (8%).
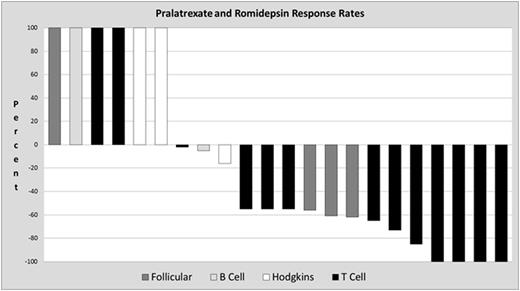
Twenty-two patients were evaluable for response, 1 patient is currently on therapy. The ORR in the total, non-PTCL and PTCL populations was 59%; 33% (no CR) and 77% respectively. Of the PTCL patients 4/13 (31%) achieved a CR, 6/13 (46%) achieved a PR, and 1 patient had stable disease. The mean duration of response (DOR) for all patients on the study (N=13) was 6.1 months (1.1 - 26.5), for the non-PTCL population (N=3) was 4.8 m (1.1-11) and for the PTCL population (N=10) was 6.55 months (range 1.6 - 26.5 +ongoing). The mean progression free survival (PFS) for all patients on study (N=26) was 4.8 m (.3 - 30.2), for the non-PTCL population (N=13) was 2.8 m (0.3-14.5), and for the PTCL population was 6.13 months (range 1.5 - 30.2 +ongoing).
Pharmacokinetic studies were performed for P and R and data for the first 15 patients is presently available for reporting. PK analyses were performed using WinNonLin® to determine Cmax and AUC. Preliminary Cmax results for P 10 mg/m2 and P 15 mg/m2 are 1810+/-1063 ng/mL and 2748+/-995 ng/mL, respectively. Preliminary Cmax results for R 12 mg/m2 and R 14 mg/m2 are 420+/-198 ng/mL and 552+/- 346 ng/mL, respectively. After infusion with P 10 mg/m2 or 15 mg/m2 PK analysis indicate AUC0-24.08h of 3616+/-1543 h*ng/mL and 4104+/-2124 h*ng/mL, respectively. AUC0-28h after treatment with R 12 mg/m2or 14 mg/m2 was 1503+/-1286 h*ng/ml and 2535+/-2560 h*ng/mL. These values are consistent with that observed for both of these drugs in previous studies.
Results from the phase I study conclude that the combination of P + R given on the QOW schedule is safe and very well tolerated. These data support the lineage specific activity of the P+R combination, which is currently being expanded to a multicenter Phase II for PTCL.
Amengual:Bristol-Myers Squibb: Research Funding; Acetylon Pharmaceuticals, Inc: Research Funding. Sawas:Seattle Genetics: Honoraria; Gilead Sciences: Speakers Bureau. O'Connor:Spectrum: Research Funding; Seattle Genetics: Research Funding; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Spectrum: Research Funding; TG Therapeutics: Research Funding; TG Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Bristol Myers Squibb: Research Funding; Celgene: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal