Abstract
BACKGROUND
Nodal peripheral T-cell lymphomas (PTCLs), except ALK-positive anaplastic large cell lymphoma (ALCL) have inferior outcomes compared with their B-cell counterparts. CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisolone) is considered the standard therapy despite consistent results showing its ineffectiveness. The addition of etoposide (E) to chemotherapy showed encouraging results in selected subgroups of patients. We performed a matched-pair analysis comparing the efficacy of EPOCH to CHOP for untreated patients with nodal PTCLs, excluding ALK-positive ALCL.
PATIENTS AND METHODS
According to the Thai Lymphoma Study Group uniform treatment project, 58 patients with nodal PTCLs including angioimmunoblastic lymphoma, ALK-negative ALCL and PTCL, not otherwise specified received conventional EPOCH regimen as frontline therapy between January 2009 and December 2015. These patients were matched to 58 patients receiving CHOP (1:1) in multicenter registry of lymphoma in Thailand between 2007-2014 by center, International Prognostic Index (IPI) and age. Patients who underwent upfront autologous stem cell transplantation (ASCT) were censored at the time of transplantation.
RESULTS
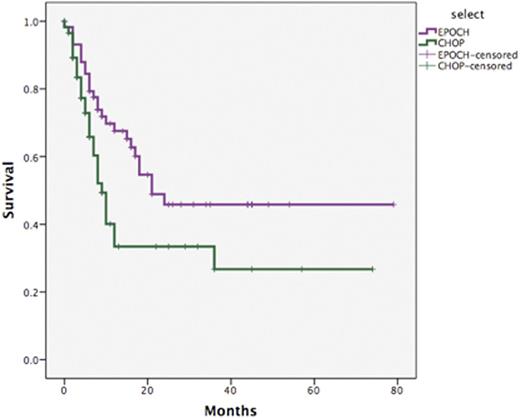
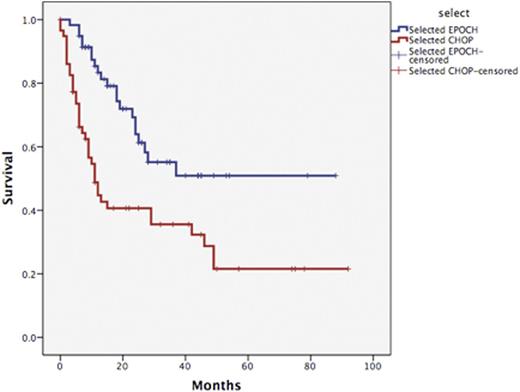
The baseline characteristics were well balanced between the two groups except a higher proportion of patients with ALK-negative ALCL in EPOCH group (24.1% vs 5.2%) (Table 1). The median cycle of chemotherapy was 6 (range, 1-8) in both groups. Four patients (6.9%) underwent upfront ASCT after EPOCH induction. The overall response rate for evaluable patients were 75.4% (complete response [CR] 57%) and 58% (CR 39%) in EPOCH and CHOP groups, respectively (P<0.0001). With a median follow up of 26 months, 4-year progression free survival (PFS) were 45.8% and 26.7% in EPOCH and CHOP group, respectively (P=0.022) (Figure 1). Projected overall survival (OS) at 4 years were 50.9% and 28.7% for EPOCH- and CHOP-treated patients, respectively (P=0.001) (Figure 2). By multivariate analysis, chemotherapy regimen was the only factor independently predicting PFS (Hazard ratio [HR] 1.82, 95% confidence interval [CI] 1.04-3.19, P=0.034) and OS (HR 2.26, 95% CI 1.28-3.97, P=0.005).
CONCLUSION
EPOCH provided superior outcomes than CHOP regimen as frontline therapy for patients with nodal PTCLS, excluding ALK-positive ALCL. The promising efficacy of EPOCH warrants further evaluation in prospective randomized controlled study.
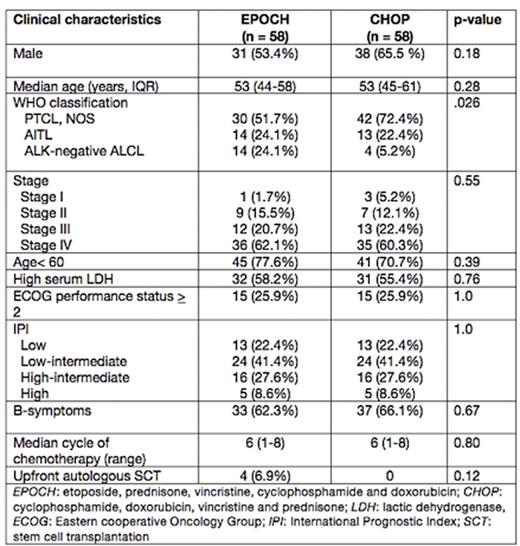
Baseline characteristics of patients with nodal PTCLs treated with EPOCH or CHOP regimens
Baseline characteristics of patients with nodal PTCLs treated with EPOCH or CHOP regimens
Kaplan Meier progression free survival of patients with untreated nodal PTCLs according to chemotherapy regimens
Kaplan Meier progression free survival of patients with untreated nodal PTCLs according to chemotherapy regimens
Kaplan Meier overall survival of patients with untreated nodal PTCLs according to chemotherapy regimens
Kaplan Meier overall survival of patients with untreated nodal PTCLs according to chemotherapy regimens
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal