Abstract
Background
Waldenstrom macroglobulinemia (WM) is an IgM-associated lymphoplasmacytic lymphoma, first described over seven decades ago. Although WM is typically a disease of the elderly, with a median age at diagnosis of ~ 67 years, approximately 10% of patients are ≤50 years (y) of age at diagnosis. Data for young patients are sparse and the few available studies demonstrate inconsistent findings, with potential overestimation of survival owing to inclusion of patients with smoldering WM. In this case-control study, we evaluate outcomes, prognostic features and impact of changing therapies in a large cohort of young symptomatic WM patients compared to matched older patients seen over the course of the past five decades.
Methods
The medical records of all WM patients seen consecutively at Mayo Clinic from 01/1960 to 10/2013 were reviewed. Of 1181 patients, 140 (11.8%) were ≤ 50 y of age at diagnosis. A cohort of patients 65 y or older at diagnosis, matched 1:1 by the time of diagnosis, served as the control population. The patients were divided into 3 groups based on the timing of initiation of therapy: Group 1 (1960-1977), Group 2 (1978-1995) and Group 3 (1996-2013). The baseline characteristics were compared. Initiation of frontline therapy was used for all time-to-event analysis using the Kaplan Meier method.
Results
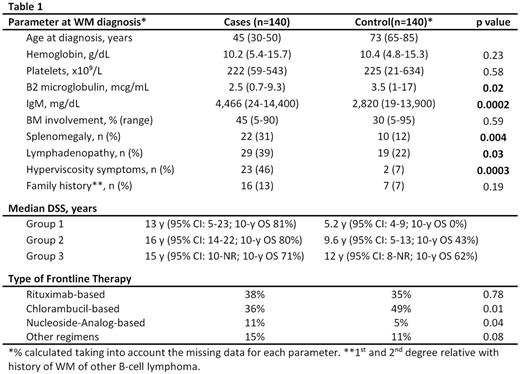
Younger patients were more likely to present with adenopathy and splenomegaly, have higher IgM levels and hyperviscosity symptoms (Table 1). The median follow-up from the frontline therapy was similar (10.7 y vs. 10 y for the control population). At the time of analysis, 91% of the deaths for the younger cases were WM-related compared to 58% in the control arm (p=0.0001). Younger patients had a better OS with a median disease-specific survival (DSS) of 15.6 y (95% CI: 13-21; 10-y OS of 77%) vs. 11 y (95% CI: 7.8-12; 10-y OS of 51%) for the older patient (p=0.0003).
Among the young patients, there was no difference in the median DSS across the 3 groups (p=0.42). However, the median DSS for the control group incrementally improved (p=0.02) over the 3 time periods (Table 1).
In the younger patients, no improvement in DSS was noted with the use of either frontline rituximab-based therapy compared to non-rituximab based regimens [median NR (95% CI: 7.6-NR) vs. 15.8 y (95% CI: 13-22) with other regimens, p=0.30], or frontline chlorambucil-based compared to non-chlorambucil based regimens [median 16 y (95% CI: 12-22) vs 15.6 y (95% CI: 12-NR) with other regimens, p=0.73]. In the control group, however, there was significant difference in DSS among patients who received frontline rituximab-based compared to non-rituximab based regimens [median NR (95% CI: 8.3-NR) vs 9.1 y (95% CI: 5.6-12) with other regimens, p=0.04], or frontline chlorambucil-based vs non-chlorambucil based regimens [8 y (95% CI: 5-12) vs 12.3 y (95% CI: 11-NR) in other regimens; p=0.001].
Conclusion
Striking differences in presentation are evident in young WM patients compared to their older counterparts. The incorporation of rituximab to the previously existing anti-WM regimens and the transition to non-chlorambucil based regimens has resulted in substantial survival gains in the older WM population over the past five decades. However, such improvement in outcome has not yet been observed in the young patients. The majority of younger patients, despite a protracted disease course, succumb to their disease.
Ansell:BMS, Seattle Genetics, Merck, Celldex and Affimed: Research Funding. Ailawadhi:Pharmacyclics: Consultancy; Novartis: Consultancy; Amgen Inc: Consultancy; Takeda Oncology: Consultancy. Reeder:Novartis: Research Funding; Celgene: Research Funding; BMS: Research Funding; Millennium: Research Funding. Kumar:Kesios: Consultancy; Janssen: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; AbbVie: Research Funding; BMS: Consultancy; Onyx: Consultancy, Research Funding; Glycomimetics: Consultancy; Millennium: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dispenzieri:Alnylam: Research Funding; Celgene: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; pfizer: Research Funding; Jannsen: Research Funding. Kapoor:Celgene: Research Funding; Takeda: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal