Abstract
Background:Lenalidomide is an immunomodulatory agent with direct and immune-mediated mechanisms of action, as well as clinical activity in NHL. Recent studies in frontline and relapsed/refractory NHL show high activity for lenalidomide plus rituximab (R2), supporting further study of this combination.
Methods: MAGNIFY (NCT01996865) is a phase IIIb, multicenter, open-label study of subjects with grades 1-3b FL (including transformed lymphoma [TL]), MZL, or MCL who received >=1 prior therapy and had stage I-IV, measurable disease (>1.5 cm). Subjects received 12 cycles of R2 induction, consisting of oral lenalidomide 20 mg/day, days 1-21 per 28-day cycle (d1-21/28) plus intravenous rituximab 375 mg/m2, days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 3, 5, 7, 9, and 11 (28-day cycles). Subjects with stable disease (SD) or better after induction were then were randomized 1:1 to R2 vs. rituximab maintenance. Stratification to the 2 maintenance arms was based on histology (FL grade 1-3b and TL vs. MZL vs. MCL), number of prior lines of antilymphoma therapy (<=2 vs. >2), and age (<65 vs. >=65 years).Maintenance R2 consisted of lenalidomide 10 mg/day, d1-21/28, cycles 13-30 plus rituximab 375 mg/m2, day 1 of cycles 13, 15, 17, 19, 21, 23, 25, 27, and 29 (Arm A). Rituximab maintenance alone was on the same schedule (Arm B). Subjects receiving R2 maintenance after 18 cycles are eligible to continue maintenance lenalidomide monotherapy 10 mg/day, d1-21/28 (optional per subject and/or investigator discretion) until disease progression as tolerated. The primary endpoint isprogression-free survival (PFS) comparing maintenance arms using a two-sided test with alpha=0.05 and HR=0.67. Secondary endpoints include safety, overall survival, and response rates. Efficacy is evaluated per modified 1999 IWG criteria and safety per NCI CTCAE version 4.03.
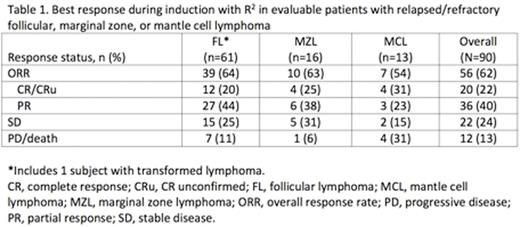
Results: As of Jan 11, 2016, 135 subjects have been enrolled, including 91 (67%) with FL, 24 (18%) with MZL, 19 (14%) with MCL, and 1 (1%) with TL. At the time of enrollment, subjects had a median age of 66 years (range, 41-91); 81% had stage III/IV disease. Subjects had received a median of 2 prior therapies (range, 1-10), with 36% refractory to rituximab (defined as SD/PD to or PR/CR for <6 months with prior rituximab). The most common prior regimens were rituximab (41%), BR (25%), R-CHP (14%), R-CHOP (11%), and R-CVP (7%). At data cut-off, 45 (33%) subjects discontinued treatment before the maintenance period. Primary reasons for discontinuation of lenalidomide and/or rituximab, respectively, were due to AEs in 16 (12%) and 14 (10%) subjects, PD in 15 (11%) subjects for either treatment, withdrawal by subject in 6 (4%) and 7 (5%) respectively, and death 3 (2%) in either treatment. In the safety population (n=124), treatment-emergent adverse events (AE) during induction that led to dose reduction/interruption of lenalidomide or rituximab occurred in 55% or 24% of subjects, respectively, mainly due to neutropenia for lenalidomide and infusion-related symptoms for rituximab. The most common grade 3/4 AEs during induction were 27% neutropenia, 9% leukopenia, 6% thrombocytopenia, and 5% fatigue. 11 subjects have died (5 due to PD, 3 AEs, 3 other). At a median duration of 23.1 weeks (range, 0.1-51.1) of induction, 90 subjects were evaluable for response. Best responses to induction were 56 (62%) subjects with ORR, 8 (9%) CR, 12 (13%) CRu, 36 (40%) PR, and 22 (24%) SD. Responses with R2 treatment were observed in all histologies (Table 1). At data cut-off, 19 subjects have completed induction and 18 have proceeded to maintenance (n=7 R2, n=11 rituximab alone). Continued study and follow-up are ongoing to enroll more subjects in the induction and maintenance arms.
Conclusions: R2 induction therapy shows favorable activity and a tolerable safety profile in subjects with advanced-stage, relapsed/refractory FL, MZL, MCL, and TL. Continued study is ongoing to determine the effect of R2 vs. rituximab maintenance following 1 year of R2 induction.
Andorsky:Gilead: Research Funding; Celgene: Consultancy, Research Funding; CTI: Research Funding. Yacoub:Incyte: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Alexion: Honoraria. Bitran:Oncology Specialists: Employment. Melear:Texas Oncology: Employment. Foon:Celgene: Employment. Rizvi:Celgene: Employment, Equity Ownership. Llorente:Celgene: Employment. Li:Celgene: Employment, Equity Ownership. Sharman:Genentech: Consultancy; Celgene: Consultancy; TG Therapeutics: Consultancy; Gilead: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal