Abstract
Background: Large case-control studies have identified common epidemiologic exposures associated with risk of AML development, including obesity [BMI ≥30 kg/m2] associated with 2-fold increase risk. However it is not known whether individual risk factors are associated with unique AML genetic lesions. We have recently identified a significant association of some common exposures including obesity with unique disease cytogenetic subgroups in a cohort of n=295 consecutive AML patients diagnosed and treated at Mayo Clinic [Cancer Epidemiol 39:1084, 2015]. Aberrant DNA methylation is a hallmark of cancer and has been reported in AML, and we therefore performed an analysis of global DNA methylation in patients in this cohort to determine whether specific epidemiologic exposures are associated with a unique pattern of DNA methylation.
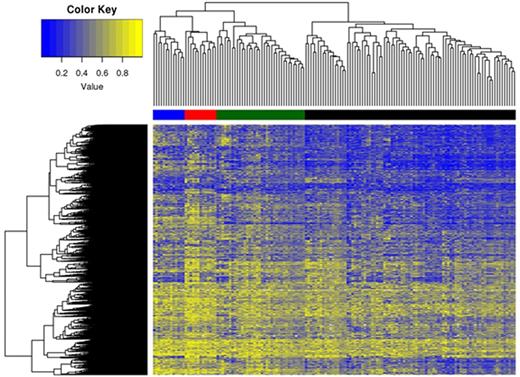
Methods: Patients in the initial cohort were previously reported. An available baseline AML bone marrow cytogenetic pellet from diagnosis was identified for n=148 patients (50.2%). Following IRB approval and using previously reported techniques [Leukemia 24:1283, 2010], leukemia DNA was isolated from the remnant diagnostic cytogenetic samples and processed on the HumanMethylation450 BeadChip array (Illumina). Individual CpG dinucleotides were assigned a Beta value (i.e. methylation value) ranging from 0 (unmethylated) to 1 (methylated). We excluded those with minor methylation changes (<0.2 between any two samples), resulting in 383,524 CpG's. For the purposes of this first analysis we have focused only on CpG's with an inter-quartile range (IQR, i.e. difference between 75th and 25th percentiles) of greater than 0.25, comprising a total of 39,657 CpG's. An unsupervised cluster analysis of differential methylation was generated ("Heatmap", Figure). Univariate and multivariable analyses were performed adjusting for age as a continuous variable to determine the association of individual exposures with unique methylation clusters (primary endpoint), and we also evaluated the association of clinical characteristics and clinical outcomes after therapy (secondary endpoint). The median age of patients was 69 yrs (range 19-89), male 65%, and Caucasian 89%. The median BMI was 27.2 (range 16.9-59.9), and n=47 patients (32%) had BMI ≥30. Standard cytogenetic risk groups were applied [favorable (n=8), normal (n=61), intermediate abnormal (n=32) and poor (n=47)].
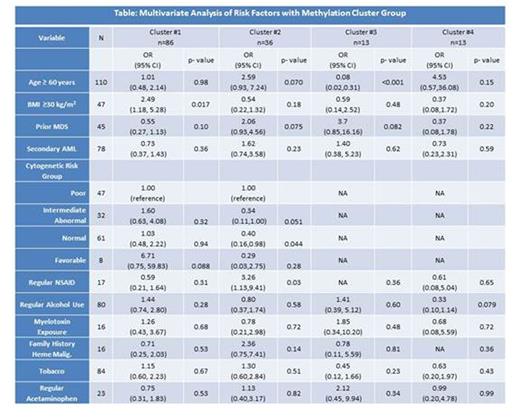
Results: We identified 4 distinct methylation clusters (Figure: Cluster #1, Black horizontal bar; Cluster #2, Green; Cluster #3, Blue; and Cluster #4, Red). Multivariate analysis of select variables is shown in Table. Patients in cluster #1 were significantly more likely to have a BMI ≥30 [Odds Ratio (OR) 2.49, p=0.017] and more likely to have favorable risk cytogenetics although this was not statistically significant [OR 6.71, p=0.088], while those in cluster #2 were significantly more likely to be have a history of regular NSAID use [OR 3.26, p=0.03] and less likely to have normal [OR 0.40, p=0.044] and possibly intermediate abnormal cytogenetics [OR 0.34, p=0.051]. Those in cluster #3 were significantly younger [median age 48 yrs, p<0.001] and were also more likely to have prior history MDS although this was not statistically significant [OR 3.7, p=0.082]. Cluster #4 was not correlated with any distinct exposure apart from possible decreased alcohol use [OR 0.3, p=0.079]. History of past or current tobacco use, regular acetaminophen use, documented myelotoxin exposure, family history, and secondary AML did not correlate with a unique methylation cluster. There was no independent association of methylation cluster with overall survival (p=0.46), although among patients receiving intensive therapy cluster #2 was associated with lower complete remission rate [OR 0.28 (95% CI 0.09-0.86), p=0.026].
Conclusion: This first analysis of AML molecular epidemiology and clinical outcome suggests that specific disease risk factors (esp. obesity and age) are associated with unique leukemia DNA methylation clusters. In addition, aberrant methylation is associated with some clinical features and with outcome after therapy, suggesting that epidemiologic risk factors may have a prognostic impact. This provides novel insight into leukemogenesis, and will guide prospective studies of AML epidemiology and outcome.
Foran:medscape: Honoraria; pfizer: Honoraria; Millennium Pharmaceuticals, Inc.: Research Funding; novartis: Honoraria; karyopharm: Honoraria; boehringer ingelheim: Research Funding; agios: Research Funding; Cellerant: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal