Abstract
Background: Pediatric acute myeloid leukemia (Pedi-AML) accounts for approximately 15% of childhood acute leukemias and the overall survival is 60-70%. Although cytogenetics provides risk stratification, most recurrent genetic events lack agents that specifically target them. Since genetic events are revealed by the expression and activation status of proteins, we hypothesized that we could define recurrent protein expression signatures that could suggest targets for individualized therapies.
Methods:Custom Reverse Phase Protein Arrays (RPPA) with 95 Pedi-AML samples and 10 normal CD34+ bone marrow samples were performed and probed with 194 antibodies to determine protein expression levels and activation status. Proteins were divided in protein functional groups (ProFnGrp) to analyze them in the context of other proteins. Progeny clustering was performed to determine the optimal number of protein clusters and principal component analysis was used to visualize the distribution between protein clusters and normal CD34+ cells. Protein networks were generated using known literature associations combined with computational derived edges from the dataset. Associations between clinical features, outcomes and protein clusters were determined. Hierarchical clustering (Figure 1) was performed on a compilation of all protein clusters into one binary matrix to identify recurrent protein expression signatures (PrSIG) that comprised similar combinations of protein constellations(PrCON). A list of proteins that were over or under expressed was made for each signature.
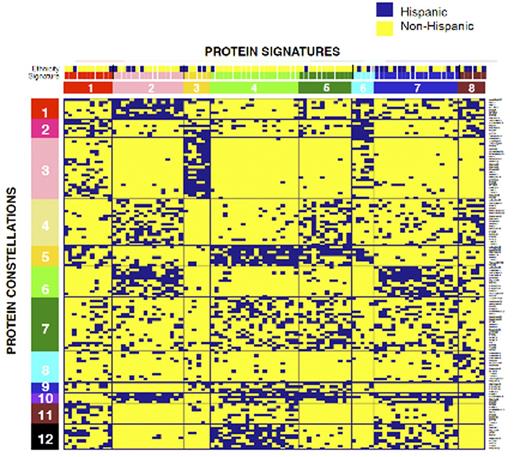
Results: For each ProFnGrp 3 to 6 distinct clusters were recognized. All but 2 ProFnGrp (Cell Cycle, Protein Kinase C) had at least one protein cluster that was similar to normal CD34+ samples and all ProFnGrp had clusters specific to the leukemic state. Protein expression levels were overlaid onto the networks and revealed distinct expression and activation states. Hierarchical clustering showed strong co-correlation between protein clusters from various ProFnGrp and suggested 12 PrCON. Patients that expressed similar recurrent combinations of PrCON compiled 8 PrSIG. Heterogeneity was observed for overall survival (OS) and complete remission duration (RD). High OS rates were seen in PrSIG 5 compared to PrSIG 1 (8/12 vs. 3/11 alive, Median NR vs. 54 wks, P=0.09) and high relapse free survival was observed for PrSIG 3 compared to PrSIG 1 (6/6 vs. 3/8 CCR, (Median RD NR vs 39 wks, P=0.04). Rearrangements of 11q23 were overrepresented in PrSIG 2 (7/16, 43.8%) and 7 (5/19, 26.3%) and underrepresented in PrSIG 4 (1/20, 5%) and 6 (0/5, 0%) (P=0.027). Complex karyotype was highly present in PrSIG 8 (4/6, 67%) compared to the overall population (16/95, 16.7%). Hispanic ethnicity was high in PrSig 7 & 8 (53 & 67%) and low in PrSIG 1, 2 4 & 5 (20%, 25%, 5%, 8%). PrSIG membership was also correlated with FAB classification (P=0.025, M0 with PrSIG 4 and M5 with PrSIG 5) as well as with WBC, blast count, HGB and PLT. As we had both Pedi-AML and acute lymphoblastic leukemia (ALL) samples on this RPPA array (see accompanying ALL abstract), this enabled comparison between protein expression levels in both diseases. Combined re-analysis suggested 12 PrSIG and 12 PrCON; most PrSIG and PrCON were dominant for ALL or AML and only 1 PrSIG and 3 PrCON showed overlap between the two diseases. Identification of significantly altered proteins for each PrSIG can be used to select targeted therapies for combination. For example, for PrSIG1 combined inhibition of RPTOR and RICTOR may be useful as both are overexpressed.
Conclusion: As hypothesized we demonstrated the existence of protein expression signatures in Pedi-AML based on strong correlations between different protein clusters. Signatures were correlated with therapeutic outcome, as well as with cytogenetics, Hispanic ethnicity, and laboratory features. Recognition of altered proteins within the signatures suggested combinations of targets that could facilitate personalized therapy. Shared protein constellations between AML and ALL indicate joint protein deregulations that could be targetable in both diseases.
Hierarchical clustering shows recurrent patterns of 12 PrCON (y-axis) that form 8 recurrent PrSIG (x-axis). The association with Hispanic ethnicity is shown.
Hierarchical clustering shows recurrent patterns of 12 PrCON (y-axis) that form 8 recurrent PrSIG (x-axis). The association with Hispanic ethnicity is shown.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal