Abstract
Background: DNA methylation in AML/MDS plays a major role in the pathogenesis of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). The major genes involved in DNA methylation in AML/MDS are IDH1 and 2, TET2 and DNMT3A. Mutations in IDH1/2 result in the production of an aberrant metabolite, 2-hydroxyglutarate, which acts as a competitive inhibitor of a-ketoglutarate and inhibits TET2 oxidation of 5-methylcytosine to 5-hydroxymethylcytosine (5hmC). Mutations in TET2 or IDH1/2 are associated with reduced levels of 5hmC and genomic hypermethylation. TET2 mutations and IDH1/IDH2 mutations are believed to be mutually exclusive. In addition, DNMT3A as a DNA methyltransferase enzyme is commonly mutated in AML/MDS and its mutation is believed to lead to hypomethylation. Understanding the interaction between these genes may influence therapy with IDH1/2 inhibitors. Toward better understanding of interaction between these genes, we analyzed the mutation profile of these genes in patients with AML/MDS.
Methods: A total of 1182 bone marrow (BM) aspirate samples were tested by the commercially available TruSight Myeloid Next Generation Sequencing Panel (Illumina, San Diego, CA). We extracted DNA from bone marrow aspirate using the QIAamp DNA Mini Kit. This NGS panel covers hot spot mutations in 54 genes. The average depth of sequencing was 10,000X.
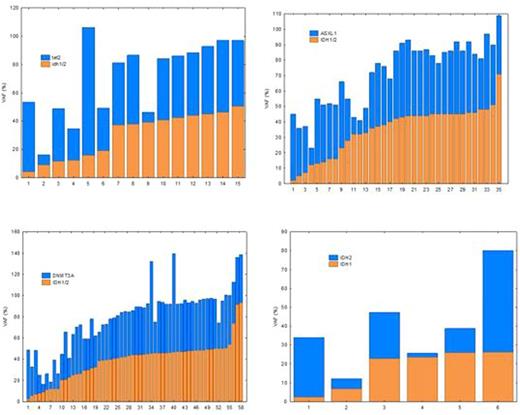
Results: IDH1/2 mutations were detected in 201 of the 1182 (17%). IDH1 was detected in 87 (7.4%) and IDH2 was detected in 120 (10.1%). This included 6 patients who had mutations in both IDH1 and IDH2. Variant (mutant) allele frequency (VAF) was significantly higher (P=0.01) in IDH2 as compared to IDH1 (median of 43.35% vs 35.0%, respectively). Thirteen patients (6.5%) had mutant VAF >50% suggesting homozygosity, 11 of which had IDH2 mutation. Two of the 6 patients with both IDH1 and IDH2 mutations had VAF <20%, raising the possibility of two independent clones. TET2 mutations were detected in 15 (7.5%) of the patients with IDH1/2 mutations. There was significant difference (P=0.03) in VAF between IDH1/2 and TET2. Nine of these patients showed comparable VAF while the other 6 patients showed completely different VAF, suggesting subclonal heterogeneity. In addition, 58 (29%) patients showed mutations in IDH1/2 and DNMT3A. While there was no significant difference in VAF between IDH1/2 and DNMT3A, VAF in IDH1/2 was >50% in 6 of these patient and in DNMT3A in 3 patients. Twenty four patients had TP53 mutation, of which 16 had IDH1 mutation and 8 had IDH2 mutation, which is disproportional with the prevalence of IDH1 mutation. There was no statistically significant difference in VAF between TP53 and IDH1/2, but 4 of these patients had both DNMT3 and IDH2 mutations and one had both IDH1 and IDH2 mutations. None of the patients with TP53 mutation had TET2 mutation.
Conclusions: IDH2 mutations may coexist with IDH1 and TET2 mutations. This co-mutation appears to be in the same clone in some patients and in a separate clone in others. The presence of VAF>50% in 6.5% of patients, which suggests homozygosity, along with co-presence of IDH1 and IDH2 and TET2 mutations suggests possible dosage effects in the biology of MDS/AML. The high rate (29%) of co-presence of DNMT3A with IDH/1/2 mutations also suggests cooperation between the two mechanisms in influencing DNA methylation and leukemogenesis. The relatively high incidence of TP53 mutation in IDH1 patients suggests that IDH1 mutation might be associated with more aggressive disease than IDH2. This data suggests that there is interaction and significant interclonal and intraclonal heterogeneity in DNA methylation genes in AML/MDS. Complete profiling of these genes is necessary for better understanding and proper prediction of clinical behavior particularly when patients treated with DNA methylation inhibitors.
Ma:Neogenomics Laboratories: Employment. Thangavelu:Neogenomics Laboratories: Employment. De Dios:Neogenomics Laboratories: Employment. Funari:Neogenomics Laboratories: Employment. Albitar:Neogenomics Laboratories: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal