Abstract
Backgrounds
Leukemic stem cells (LSCs) have emerged as one of reasons for relapse to chemotherapy. To eradicate these quiescent LSCs is of great importance. Administration of granulocyte colony stimulating factor (G-CSF) with low-dose chemotherapy, as a priming strategy, has shown satisfactory effect in acute myelocytic leukemia treatment, especially in elder patients. It is important to investigate the specific signaling pathways and explore useful biomarker to monitor clinical response to therapy. MiR-146a and its downstream putative targets CXCR4 and Smad4 may sustain quiescent LSCs in bone marrow niches. The objective of this study is to further explore the possibility of miR-146a as a marker for sensitivity detection to G-CSF priming chemotherapy.
Patients and Methods
MiR-146a over-expression (OE), knock-down (KD) and empty virus (EV) HL-60 cells were constructedin vitro. Leukemia cells were pre-treated with G-CSF for 48h or not. MiR-146a, p50, p65, CXCR4 and Smad4 expression levels were detected. Human stromal cell line HS-5 and HL-60 cells were co-cultured to simulate interaction between bone marrow microenvironment and leukemia cells. Chemotaxis, cell viability, cell cycle and apoptosis were estimated individually. Relationship between miR-146a expression and prognostic factors were analyzed in 46 new diagnosis AML patients, including overall survival (OS) rates, relapsed-free survival (RFS) rates and complete remission (CR) rates.
Result
MiR-146a-OE cells showed lower CXCR4 and Smad4 levels than miR-146a-EV cells (p=0.011 and 0.01), while miR-146a-KD cells showed increased CXCR4 and Smad4 expression (p=0.0002 and 0.001), both in mRNA and total protein levels. Higher miR-146a expression was observed in G-CSF pre-treated group. p50 and p65 mRNA and total protein levels were both elevated in response to G-CSF treatment (p<0.01). Meanwhile, the amount of cells migrating to medium containing SDF-1a or HS-5 cells decreased significantly. More cells turned into G1 phase with accelerated proliferative rates, and more apoptotic cells were detected in response to cytarabine treatment.
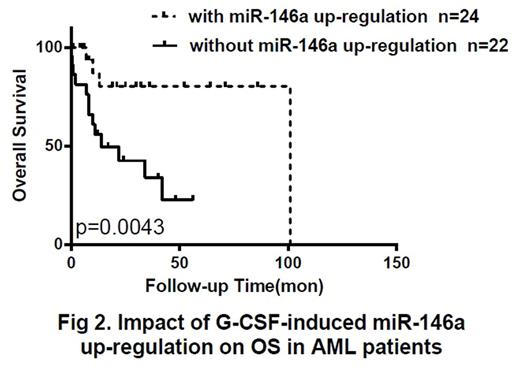
Taking median miR-146a value (3.085) as boundaries, patients with high miR-146a expression at diagnosis (n=23) had longer 2-year OS rates (56.5% vs 26.1%, p=0.042), and longer 2-year RFS rates (52.2% vs 13%, p=0.044) than low-expression group (n=23) (Fig 1). After first course of CAG regimen treatment, sensitive respondents with significantly increased miR-146a (n=24) showed longer OS (83.3% vs 40.9%, p<0.01), longer RFS (62.5% vs 22.7%, p=0.032), and higher CR rates (70.8% vs 40.9%, p=0.042) than those with no molecular response (n=22) (Fig 2). According to their response to G-CSF treatment, we divided patients with low miR-146a expression at diagnosis into 2 subgroups. It was proved that significant miR-146a up-regulation after G-CSF treatment promoted more patients into CR courses with longer OS (80% vs 23.1%, p<0.01) and RFS (50% vs 15.4%, p=0.023).
Conclusions
1. CXCR4 and Smad4 are both downstream targets of miR-146a.
2. G-CSF up-regulates miR-146a expression via activating upstream NF-KB signaling pathway, thus altering a series of biological processes including migration, proliferation and cell cycle of leukemia cells, and eventually sensitized them to chemotherapy.
3. High miR-146a levels at diagnosis might predict better outcomes.
4. Patients with significantly increased miR-146a levels after G-CSF treatment often have longer OS and RFS rates.
5. MiR-146a might be a novel biomarker for evaluating clinical prognosis and treatment effect.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal