Abstract
Background: APL is the most curable leukemia with reported survival above 90% in many large co-operative group studies. These excellent outcomes are not seen in elderly patients where early deaths in trials are 10-18%. Population based registry data shows that ED rate in elderly APL patients is even higher with reported incidence of 24-50%. We conducted a prospective trial using a set of simplified treatment guidelines along with support from a dedicated team of APL experts. Herein, we report outcomes of patients >60 years treated within the prospective trial. We compared these to our institutional experience prior to implementation of the trial.
Methods: The protocol consisted of a two page simplified treatment algorithm with emphasis on preventing bleeding, infection and differentiation syndrome (DS); the most common causes of ED. A network of Leukemia treatment centers was established by visiting most of the leukemia treatment centers in Georgia, South Carolina and neighboring states. Communication was established between the treating community oncologist and APL expert early in the diagnosis and was continued until the end of induction. Five other large leukemia treating centers in Georgia and South Carolina were part of the study and IRB approval was obtained. After 6 months of intense publicity, the study was implemented in 7/2013 and patients were prospectively accrued. Induction deaths of elderly APL patients in our study were compared to historical results at our institution (with IRB approval for retrospective review) prior to the initiation of this program. The study was funded by the Leukemia Lymphoma Society. Statistics are descriptive.
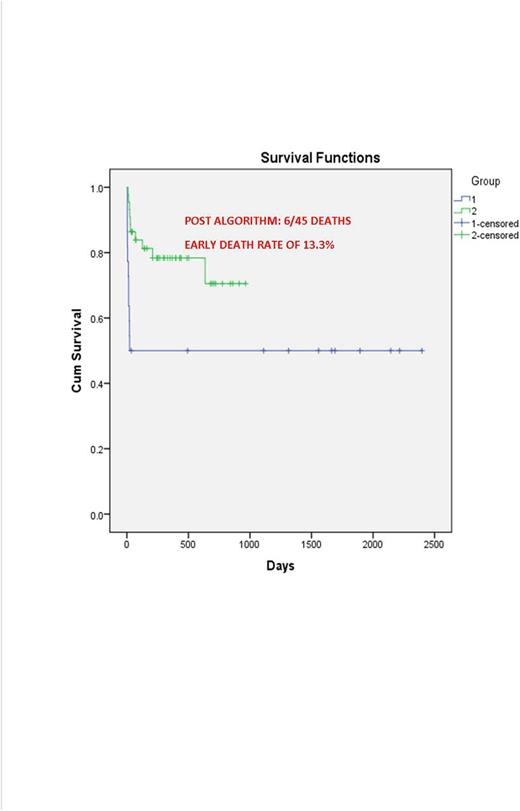
Results: Between 1/2007 and 5/2016, we treated or co-managed a total of 70 patients above the age of 60 years. 23 were in the early group (EG) and 47 after 7/2013 in the new group (NG). Comparing the EG and NG patients, median age was 66 years (range 61-80) vs 69 (61-83 years), median WBC was 1.9 vs 2.3/mm3 (range 0.3-132 vs 0.3-38), male to female ratio was 1:2 and 1:1 respectively. Risk stratification based on WBC counts at presentation (> vs <10,000/mm3) showed 73% low risk in EG vs 93 in NG. All patients were treated in our institution in EG while in the NG 13 (28%) were treated in our institution and 34 (72%) were managed in 18 other centers. 20 patients (42.5%) were treated in large centers and 27 (57.5%) in community centers with support from APL experts. In the EG, induction consisted of Al-trans retinoic acid (ATRA) alone (n=5, 21.7%), ATRA with ATO (n=5, 21.7%) and ATRA with anthracyclines (AC) (n= 13, 56.6%). In the NG, induction was ATRA alone (n=2, 4.3%), ATRA with chemotherapy (n= 2, 4.3%) and ATRA with ATO in others (n=43, 91%). In the NG doses of ATRA and/or ATO were reduced in a majority of the patients. Complications during induction for the EG vs. NG were as follows: bleeding [n=4 (17.3%) vs 5 (11%)], infection [n=13 (56.6%) vs 10 (22%)] and differentiation syndrome [n=13 (56.6%) vs 18 (40%)]. In the EG 12 patients (52%) died during induction. However 4 patients were discharged to hospice immediately after presentation and 1 patient refused care bringing the induction mortality to 7/18 (39%). NG had 8 induction deaths, however one was a Jehovah's Witness who refused transfusions and the other we were consulted on 12 days after diagnosis with multi-organ failure. The mortality rate in the NG was 6/45 (13.3%). 4 patients in the NG experienced late deaths from second cancer (n=1), relapse (n=1) and non APL related deaths (n=2). With a median follow up of 267 days in the NG, overall survival was 77.8% compared to 42% in the EG.
Conclusions: By our observation, elderly patients tolerate full doses of ATRA and ATO poorly during induction. ATRA/ATO at reduced doses is better tolerated and use of a simplified treatment algorithm with expert support has the potential to decrease induction mortality (13.3%) and improve population wide survival (77.8%) (1 year survival in patients >60 years based on Swedish Registry data ~ 37%) in elderly APL patients.
Kota:Leukemia Lymphoma Society: Research Funding; Ariad Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Heffner:Millennium: Research Funding; AbbVie: Research Funding; Pharmacyclics: Research Funding; Celgene: Research Funding. Stuart:Bayer: Research Funding; Celator: Research Funding; Incyte: Research Funding; Agios: Research Funding; Sunesis: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding; Astellas: Research Funding. Gerber:Spectrum: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Alexion: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Grunwald:Forma Therapeutics: Research Funding; Medtronic: Equity Ownership; Ariad: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Alexion: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jillella:Leukemia Lymphoma Society: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal