Abstract
Acute Myeloid Leukemia (AML) is the most common type of acute leukemia in adults and the second in children. The overall survival is less than 35% and 60% for adults and children respectively. Activating mutations of FLT3 are now recognized as the most common molecular abnormality in this disease, and the poor prognosis of patients harboring these mutations renders FLT3 an obvious target of therapy. Although different tyrosine kinase inhibitors (TKI) have been used for this purpose, the ability of these drugs to extend progression-free and overall survival in this patient population is limited by drug resistance. This strategy could be improved by rationally combining TKIs with other agents. In this work, we have explored by phosphoproteomics the alternative pathways activated after TKI treatment in vitro and ex vivo.
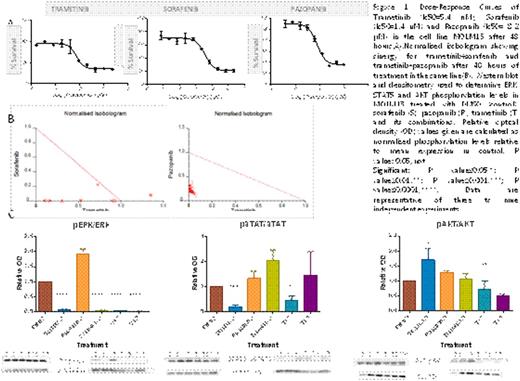
The phosphoproteome profile of the bone marrow from a FLT3-AML patient before and after TKI treatment, studied by LC-MSMS after IMAC enrichment, suggested the activation of Ras-Raf-MEK-ERK1/2 pathway as a possible mechanism for TKI resistance, which could be avoided by dual inhibition using the MEK inhibitor trametinib. Therefore, we characterized the effect of trametinib in combination with the TIK pazopanib and sorafenib by the in vitro cell viability assay using WST8in the FLT3-ITD AML cell line MOLM13. As it is presented in figure 1a, trametinib showed an IC50 value in the low-nanomolar range (5.4 nM) and this MEKI produced a strong synergy (0.5<Ci) with the two TKI tested (figure1b). Moreover, when we analized the activation of the three main pathways downstream the FLT3 receptor by western blot (figure 1c), we observed that the combination of trametinib with both pazopanib and sorafenib showed a complete inhibition of phospho-ERK1/2 compared to the DMSO control (P≤0.0001). Interestingly, we also observed some differences between the two combinations: while trametinib in combination with sorafenib inhibited STAT5 phosphorilation (P≤0.05), the MEKI combination with pazopanib produced a significant decrease on phospho-AKT levels (P≤0.01). In conclusion, we provide preclinical evidence that combining a TKI, such as sorafenib or pazopanib, with a MEKI, such as trametinib, is a rational and efficacious treatment regimen for AML. As trametinib has previously shown good results when combined with pazopanib in clinical trials for other kind of tumors, we expect similar results in AML. On the other hand, trametinib+sorafenib could offer an optimal combination in those patients with elevated levels of phospho-STAT5, as it has been described for patients which present FLT3-ITD mutations.
M.L. holds a postdoctoral Fellowship of the Spanish Ministry of Economy and Competitiveness (FPDI-2013-16409) y ML.M. holds a Fellowship of the Spanish Ministry of Education, Culture and Sport (REF-91442).
Martínez-López:Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal