Abstract
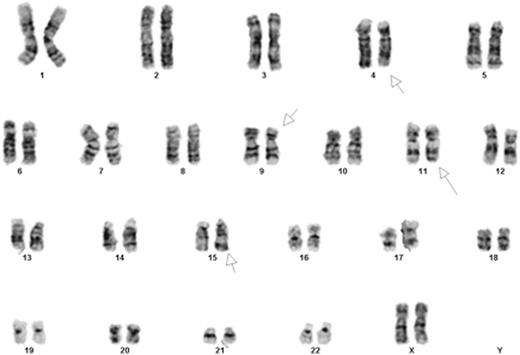
Introduction: We describe for the first time, a novel chromosomal translocation in AML. The cells bearing this translocation proliferated rapidly following stimulation with ATRA in vitro. A 30-year old woman presented with monocytic AML. Karyotype revealed: 46,XX,t(9;11)(p22;q23)[13]/46,XX[7]. Mutational analysis showed an activating mutation in NRAS (c.34G>A; p.G12S). She achieved remission with standard induction therapy followed by cytarabine consolidation in the absence of an optimal donor. Following a disease free interval of 8 months, she relapsed. Karyotype at relapse: 46,XX,t(4;15)(q31;q22),t(9;11)(p22;q23)[20] (Figure 1). Mutational analysis revealed no new mutations. In the setting of chemotherapy refractory disease, she was enrolled on a phase I clinical trial combining escalating doses of TCP (tranylcypromine, Parnate¨) with fixed doses of ATRA (NCT02273102). The patient died from rapid disease progression shortly after her first cycle of therapy. Unexpectedly, we observed a rapid proliferation in the patient's blasts following treatment with ATRA in vitro. We hypothesized that the novel t(4;15) translocation was involved in the regulation of retinoic acid (RA) signaling, and may have contributed to the rapid disease progression observed in this patient when she was treated with ATRA. To this end, we fully characterized the fusion gene and created a cell line bearing the translocation. Using these cells, we will further elucidate the mechanisms accounting for a rare and potentially clinically relevant effect of ATRA, in vitro.
Methods: Cytogenetics and molecular-cytogenetic techniques, next generation sequencing (WES), RNA-seq, RT-PCR, and direct Sanger sequencing were used to map the chromosomal translocation. Cell proliferation and Annexin V apoptosis assays were used to test the effects of ATRA and various retinoic acid receptor (RAR) agonists and antagonists in vitro. Cells were treated with ATRA, RARα, and RARγ agonists and antagonists for 72 h and cell proliferation and apoptosis were measured with CellTiter-Glow (Promega)¨ luminescence viability assay and Annexin V/PI staining (FACS), respectively.
Results: We mapped the t(4;15)(q31;q22) at the single nucleotide level, and discovered a novel fusion of TMEM154 (4q31.3) and RASGRF1 (15q24.2) genes at both the DNA and RNA level. The fusion protein included exons 1-6 from TMEM154 and exons 15-24 from RASGRF1. RASGRF1 (Ras protein specific nucleotide releasing factor 1) activates Ras by catalyzing the exchange of Ras-bound GDP for GTP. We determined that TMEM154 (transmembrane protein 154) is regulated by RA based on gene expression profiling. This particular translocation appears to result in a proliferative advantage in AML cells treated with RAR agonists such as ATRA, even at physiological levels (10 nM) (Figure 2). In MLL-rearranged cell lines without t(4;15), ATRA has a neutral or anti-proliferative effect, suggesting that the t(4;15) product interacts with the RA pathway to induce proliferation of AML blasts. We hypothesize that by increasing expression of TMEM154-RASGRF1, ATRA drives proliferation through Ras signaling. Of note, RAR antagonists inhibited proliferation and induced cell death (Figure 2). Also, treating the cells with MAP Kinase and Ras inhibitors consistently diminished the proliferative effect of ATRA. Updated studies shedding light on the mechanism of interaction between RA signaling and the TMEM154-RASGRF1 fusion protein will be presented.
Conclusions: Our study for the first time identifies t(4;15) as a novel and pathogenic translocation in AML. This rare event may confer a proliferative advantage in the presence of ATRA and caution is advised should patients with this lesion be considered for treatment with ATRA.
Conventional karyotyping showing 46,XX,t(4;15)(q31;q22),t(9;11)(p22;q23).Chromosome analysis was performed on 20 G-banded metaphase cells from multiple unstimulated cultures. Both translocations were present in all cells examined.
Conventional karyotyping showing 46,XX,t(4;15)(q31;q22),t(9;11)(p22;q23).Chromosome analysis was performed on 20 G-banded metaphase cells from multiple unstimulated cultures. Both translocations were present in all cells examined.
ATRA and other RAR agonists increased proliferation in t(4;15) AML cells. Primary cells from a patient with t(4;15) AML were treated with ATRA, RARα (AM80 and 195183), and RARγ (205327) agonists, and RARα (196996), RARγ (205728) and dual (194310) antagonists. Cell proliferation was determined by CellTiter-Glo¨ luminescent cell viability assay (Promega) after 72 h of treatment.
ATRA and other RAR agonists increased proliferation in t(4;15) AML cells. Primary cells from a patient with t(4;15) AML were treated with ATRA, RARα (AM80 and 195183), and RARγ (205327) agonists, and RARα (196996), RARγ (205728) and dual (194310) antagonists. Cell proliferation was determined by CellTiter-Glo¨ luminescent cell viability assay (Promega) after 72 h of treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal