Abstract
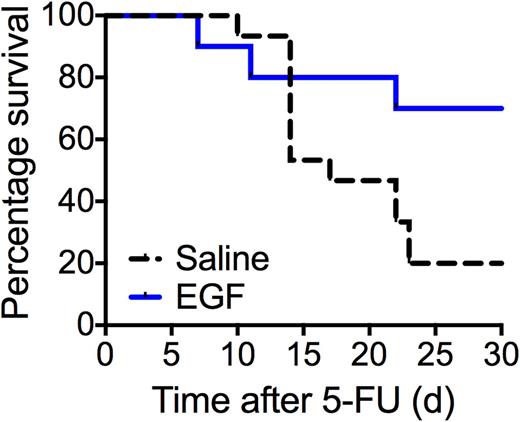
Hematopoietic stem cells (HSCs) reside in specialized bone marrow microenvironments adjacent to endothelial cells (BM ECs). BM ECs regulate both HSC self-renewal and regeneration by expressing soluble growth factors such as epidermal growth factor (EGF). EGF accelerates HSC regeneration following ionizing radiation injury (Doan et al., Nat Med, 2013), though its role in HSC reconstitution after chemotherapy is not yet defined. At 24 hours following administration of 5-fluorouracil (5-FU), the expression of EGF receptor is increased 10-fold (p<0.0001) compared to untreated control mice within ckit+Sca-1+Lineage- (KSL) cells. EGFR expression is preferentially induced 6.8-fold in KSL cells compared to whole bone marrow cells after 5-FU (p=0.01). When C57Bl6 mice are treated with a single injection of 150 mg/kg 5-FU followed by daily injections of EGF for 4 days, they demonstrated preserved BM cellularity (p=0.0003), increased BM EC density (p=0.002), and contained higher levels of donor engraftment at 16-weeks post-secondary transplantation in competitive repopulating assays (mean 21.9% vs 52.2%, p=0.0005) compared to saline-treated control mice. Following a lethal dose of 5-FU, EGF-treated mice displayed 70% survival compared to 20% survival in saline-treated control mice (p=0.04). These results suggest that EGF signaling may accelerate both hematopoietic progenitor and stem cell reconstitution following myelosuppressive and lethal-dose chemotherapy.
Since the pro-apoptotic gene Bax is increased 2.8-fold in BM lin- cells following 5-FU compared to cells from untreated mice (p<0.0001), we sought to determine whether chemo-protection of BM ECs via deletion of Baxwould accelerate HSC reconstitution. We employed a genetic mouse model with Cre recombinase to delete Bax specifically in VECadherin-expressing ECs (VECadherinCre;BaxFL/+ and VECadherinCre;BaxFL/FL mice). These mice displayed no differences in hematopoiesis at baseline. When C57Bl6 KSL cells are in non-contact co-cultures with BaxFL/+ BM ECs and treated with 5-fluorodeoxyuridine monophosphate (FdUMP) + EGF for 48 hours, they displayed both increased colony-forming cells (CFCs) and decreased annexin+ cells compared to control cultures (p=0.001 and p=0.03, respectively). Conversely, when C57Bl6 KSL cells are cultured with BaxFL/FL ECs and FdUMP + erlotinib, a tyrosine kinase inhibitor that neutralizes EGFR signaling, they displayed decreased CFCs and increased annexin+ cells compared to control cultures (p=0.001 and p=0.0003, respectively). These data suggest that chemo-protection of BM ECs by deletion of Bax in vivo results in accelerated hematopoietic recovery.
Following 5-FU, EGF promoted cell cycling with increased Ki67+ cells and cells in interphase (mean 4.8% vs 16.1%, p=0.03). Moreover, EGF decreased annexin+ cells within the KSL population both in vitro following FdUMP (mean 26.3 % vs 17%, p=0.002) and in vivo following 5-FU (mean 21.5% vs 11.5%, p<0.0001). Concordant with these results, KSL cells treated with FdUMP + EGF displayed lower levels of double-strand DNA (dsDNA) breaks, measured by γH2AX, compared to control cultures (mean 39% vs 16%, p<0.0001). Since EGF can repair dsDNA breaks via non-homologous end joining recombination, we measured the levels of phosphorylation of DNA pk cs. Following 24 hours with FdUMP and 15 minutes with EGF, KSL cells displayed a 4.8-fold increase in phospho-DNA pk cs (T2647) compared to control cultures (p=0.04). Finally, we sought to determine whether EGF could accelerate HSC reconstitution via the granulocyte colony-stimulating factor (G-CSF) receptor. Following 5-FU + EGF, G-CSF-R expression is increased 3.6-fold in mRNA levels (p<0.0001) and by flow cytometric analysis (mean 23.9% vs 42.4%, p=0.003) compared to 5-FU-treated mice. These results suggest that EGF may be a potent HSC growth factor and may exert its effect at least in part via G-CSF receptor signaling.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal