Abstract

Background
Massive transfusion (MT) has been defined as transfusion of ≥10 red blood cell units in 24 hours or half the blood volume (≥5 units) in 4 hours. Launceston General Hospital is a 330 bed hospital in regional Australia and we developed critical bleeding - massive transfusion protocol in accordance with the National Blood Authority (Australia) patient blood management guidelines. In 2012 we developed a system by which blood samples on all the MT patients are flagged as 'super-urgent', and our lab staff will perform assays for full blood counts, coagulation profile, crossmatch and biochemistry immediately and communicate to the clinicians, providing a rapid turnaround time. We sourced a dedicated blue specimen bag for MT for easy identification of blood specimens (both hand delivered and via Lamson tube) by the lab staff. We postulate that this system is both practical and achievable in hospitals dealing with MT and has the potential to improve patient outcomes and reduce wastage of blood components, when compared to empirical use.
Methods
We performed a retrospective analysis on the data collected for patients who underwent MT for 4 years between January 2012 and December 2015. All the patients who had critical blood loss requiring ≥10 units of packed red cells within a 24 hour period or ≥5 units within a 4 hour period were included in our study. The clinical and lab parameters were collected from the MT data entry sheets. Full blood counts (FBC) were performed by Sysmex XE-5000 and coagulation assays on the plasma (PT, aPTT and fibrinogen) by Sysmex CS2100i analyser. The turnaround time (TAT) from specimen reception at the lab to authorization of results for each of the super-urgent episodes sent in blue specimen bags (FBC and coagulation assays) were extracted from the laboratory information system. Clotted and hemolysed specimens were excluded. Data on blood components transfused and clinical outcomes across different clinical settings were reviewed. Statistical analyses were performed with Graph-Pad Prism software (Version 6.01, San Diego, CA).
Results
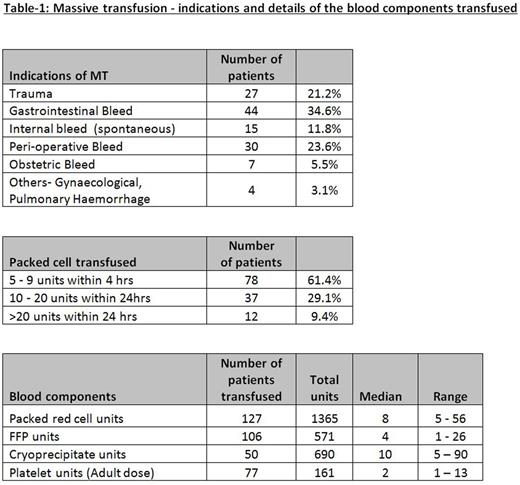
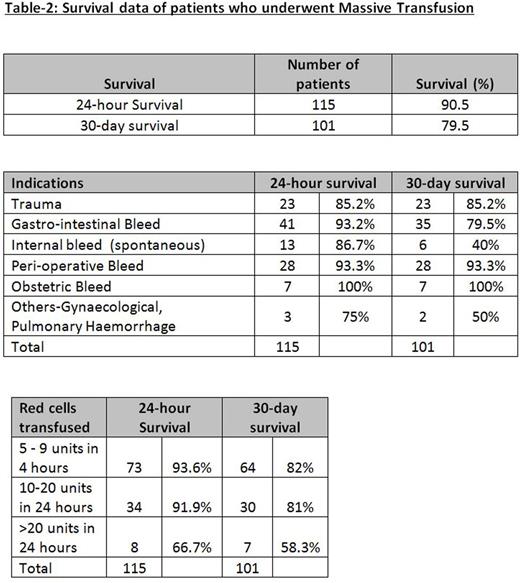
A total number of 127 MT episodes in 125 patients were included in the study. The median age was 63 years (range: 20-89) with 73 males (58%) and 52 females (42%). Table1 presents the details of blood component management. For the 127 MT episodes, there were a total of 265 super-urgent FBC and 285 super-urgent coagulation assays. We observed that median TAT for super-urgent tests were significantly lower than the median of average TAT calculated for urgent tests performed in the lab on the same days of MT for both FBC (10 minutes [IQR: 6-16] vs 31 minutes [IQR: 27-36], P<0.0001) and coagulation assays (25 minutes [IQR: 20-33] vs 45 minutes [IQR: 39-49], P<0.0001). Of the 127 MT episodes, a total of 115 patients were alive at 24 hours (90.5% survival) and 101 patients at 30 days (79.5% survival). Table-2 presents the survival data for different categories.
Conclusion
Many current MT protocols are based on empirical therapy to maintain the ratio of packed red cells to FFP and packed red cells to platelets. Short TAT and rapid communication of lab results to the clinicians during MT episodes will provide real-time estimation of the coagulation system and aid in more appropriate usage of blood components when compared to empirical administration. In our cohort of MT patients, we were able to achieve shorter TAT for super-urgent than urgent FBC and coagulation assays. Point-of-care (POC) hemostasis testing devices are currently used in MT settings; however their main limitations are accuracy, testing performed by non-lab-trained personnel and quality assurance. Hence lab FBC and coagulation tests with rapid TAT can provide better directed information for management of MT. We also observed that mortality rates in our cohort were lower than seen in comparable studies. Based on our retrospective analysis, we postulate that rapid TAT of lab results contributed to the low mortality rates by assisting our clinicians in the rational management of blood components and avoidance of under-treatment or over-treatment. This is a pilot study which will form the basis for prospective randomised studies to fully evaluate the true utility of the system. We believe this pragmatic solution will contribute to improved patient outcomes and optimise the appropriate use of precious resources in the setting of critical bleeding massive transfusion.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal