Abstract

Background:Thrombotic thrombocytopenic purpura (TTP) is a rare but deadly thrombotic microangiopathy (TMA) that is caused by an acquired deficiency in the ADAMTS13 enzyme. The PLASMIC clinical scoring system was developed and validated in 2014 by the Harvard TMA Research Collaborative in order to determine the pretest probability of severe ADAMTS13 deficiency in cases of suspected TTP. We studied the role of the PLASMIC score in guiding use of the ADAMTS13 activity assay and assessed the cost effectiveness of a score-based diagnostic approach to patients with TMA compared to two strategies currently in use within our consortium.
Methods:We utilized an expanded dataset from the Harvard TMA Research Collaborative consisting of all consecutive cases of TMA at three large academic medical centers for which an ADAMTS13 assay was sent between 2004-2015 (n=647). To investigate trends in ADAMTS13 testing over time, we compared the experience at two centers (A and B) with a liberal ADAMTS13 testing policy against a third center in our consortium (C) that carries a more restrictive policy requiring pre-approval by the blood transfusion service. Cost savings analysis was subsequently performed to assess the potential impact of an algorithm incorporating the PLASMIC score for clinical decision support in the workup of these patients.
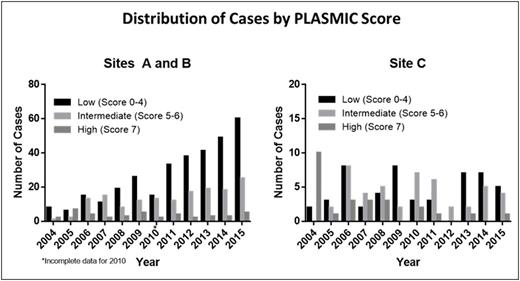
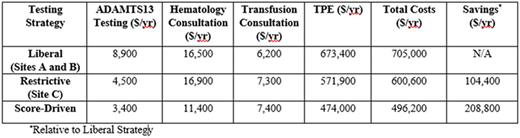
Results:At Sites A and B, we observed after adjusting for changes in inpatient volume that the quantity of ADAMTS13 tests increased greater than eight-fold during the study period (from 11 per 100,000 admissions in 2004 to 94 per 100,000 admissions in 2015, P <0.05). Despite this increase in testing, the average number of patients diagnosed with severe ADAMTS13 deficiency remained steady (5.48 cases per 100,000 admissions per year from 2004-2009 versus 4.67 cases per 100,000 admissions per year from 2010-2015, P=0.93). Furthermore, stratification of patients by PLASMIC score revealed that low-risk cases (score 0-4) have accounted for the majority of ADAMTS13 testing over time, comprising an average of 59% (range: 39-72%) of tests sent each year (see Figure). By contrast, over the same period of time, Site C did not show a significant increase in ADAMTS13 testing (24 per 100,000 admissions in 2004 to 22 per 100,000 admissions in 2015, P=0.82) and had a steady number of patients with severe ADAMTS13 deficiency (7.96 cases per 100,000 admissions per year from 2004-2009 compared to 5.53 cases per 100,000 admissions per year from 2010-2015, P=0.83). Site C also had a lower average proportion of patients with low-risk PLASMIC scores who received ADAMTS13 testing each year (39%, range: 0-70%, P=0.004 for comparison with Sites A and B). No patient with a low risk score in our registry was found to have severe ADAMTS13 deficiencybetween 2004-2015,and we have previously shown that therapeutic plasma exchange (TPE) does not improve mortality or hospital length of stay in the subgroup of TMA cases without severe ADAMTS13 deficiency. Under the score-driven diagnostic approach, patients with a low-risk PLASMIC score would not receive upfront ADAMTS13 testing, expert consultation, or TPE and instead be closely observed while worked up for alternative causes of TMA. Consortium-wide cost savings analysis demonstrates that risk stratification of patients by PLASMIC score to guide use of both testing and therapy would have decreased total costs by 30% ($208,800) in the most recent year studied (2015, n=100 cases of suspected TTP) without any projected change in outcomes by preventing unnecessary testing ($5,500), hematology and transfusion medicine consultations ($5,900), and TPE treatments ($199,600) (see Table).
Conclusions:Taken together, our results demonstrate that a significant number of patients in our consortium who are at minimal risk for TTP nevertheless undergo ADAMTS13 testing and receive expert consultation and/or TPE. An approach incorporating upfront application of the PLASMIC clinical scoring system to risk stratify patients with suspected TTP may help enhance the cost effectiveness of diagnosing and managing these cases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal