Abstract
Introduction: Depending on the conditioning intensity and graft source, mosthematopoietic stem cell transplant(HSCT) patients invariably experience a cytopenic phase of at least 2-3 weeks and require transfusion support until engraftment. All transfused blood components post-transplant need to be gamma irradiated to prevent transfusion associated graft versus host disease (TA-GVHD). Platelet component (PC) manufacturing methodologies differ around the globe. Currently, Hong Kong HSCT patients are supported with individual platelet rich plasma (PRP) PCs without leukocyte filtration (NLF), tested by short-term aerobic cultures for bacterial contamination (STABC), and gamma irradiated. When new PC manufacturing methodologies are considered for adoption, it is important to assess the immediate and longer term effects on transfused patients. Amotosalen+UVA light (A-UVA) pathogen reduction technology (INTERCEPT™ Blood System, Cerus Corporation, Concord, CA) inactivates bacteria, viruses, parasites and leukocytes by efficient covalent adduct modification of DNA and RNA. Treatment with A-UVA prevents TA- GVHD in an animal model, inhibits clonal T cell proliferation, prevents allogeneic antigen stimulation in mixed lymphocyte reactions, and inhibits transcription mediated cytokine production and early activation antigen expression (Corash et al, BMT 2004). The common strategy of prescribing gamma irradiated PC only for suspected "high risk" patients is sub-optimal, as failure to identify recipients at risk for TA-GVHD was responsible for 50% of reported cases (Kopolovic et al. Blood 2016). Treatment of PC with A-UVA addresses two key issues for HSCT patients: bacterial contamination, and universal prevention of TA-GVHD. Here, we compared the clinical support of HSCT patients with conventional PCs (C-PC) vs. an equivalent HSCT group supported with A-UVA PCs (I-PC) using a sequential patient cohort design within a single HSCT clinical center.
Methods: I-PCs were prepared from 5 pooled ABO-matched, NLF buffy coat PCs in platelet additive solution and treated with A-UVA replacing gamma irradiation and bacterial detection. C-PCs were prepared from 5 ABO-matched, STABC-negative, NLF PRP PCs, and treated with gamma irradiation. Each patient could receive up to 5 PC transfusions. The primary efficacy endpoint was the 1-hour corrected count increment (CCI) and the primary safety endpoint was the proportion of patients (P) with acute transfusion reactions (ATR). Follow-up data on mortality, hematopoiesis engraftment, and immune status were collected up to 100 days post HSCT.
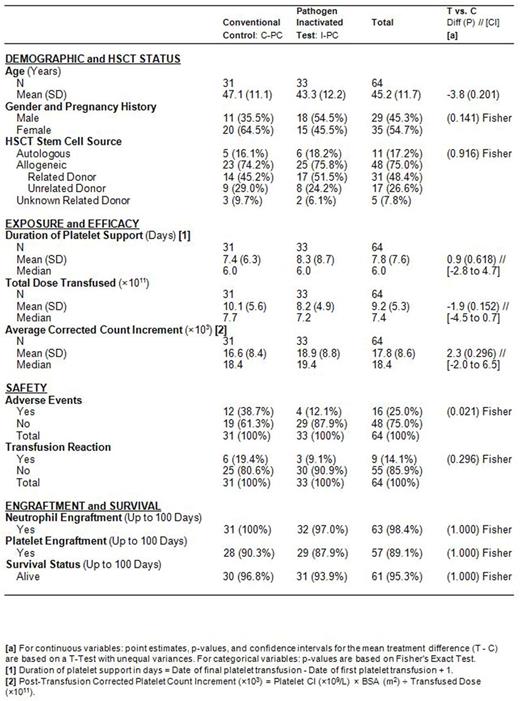
Results: Patient demographics and type of HSCT were similar between patient cohorts. 33 patients received 76 A-UVA PCs and 31 patients received 89 C-PCs. The mean days of platelet support (p=0.618) and mean 1-hour CCIs per patient averaged for all transfusions were comparable (p=0.296) between the I- PC and conventional C-PC cohorts (Table). The proportion of patients with AEs was lower (p=0.021) for the I-PC group (Table), and no related SAEs were observed during the entirety of trial. Survival at 100 days post HSCT and rates of remission were similar between the cohorts (Table). The ATR rate trended lower, although not significantly different (p=0.296), in the I- PC group (Table). Follow-up data showed that the patients had comparable neutrophil and platelet engraftment (Table) with comparable immune system reconstitution by 100 days post HSCT. No cases of TA-GVHD were observed in either cohort.
Conclusions: A-UVA-treated PCs prepared without LF, gamma irradiation, and bacterial detection can replace C-PCs for support of HSCT patients resulting in comparable post transfusion CCI responses and short and intermediate term clinical outcomes, while offering additional protection against transfusion transmitted bacteria and emerging or untested pathogens.
Sim Pui Yin:Cerus Corporation: Other: Investigator sponsored trial. Leung Yuk Yan:Cerus Corporation: Other: Investigator sponsored trial. Lam Chun Kit:Cerus Corporation: Other: Investigator sponsored trial. Tsoi Wai:Cerus Corporation: Other: Investigator sponsored trial. Lee Cheuk:Cerus Corporation: Other: Investigator sponsored trial. Lie Kwok Wai:Cerus Corporation: Other: Investigator sponsored trial. Huang:Cerus Corporation: Employment. Rico:Cerus Corporation: Employment. Lin:Cerus corp: Employment. Corash:Cerus Corporation: Employment. Stassinopoulos:Cerus Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal