Abstract
The risk of venous thromboembolism (VTE) is increased in individuals with cancer, particularly in those with multiple myeloma. The immunomodulatory agents (IMiDs) thalidomide, lenalidomide and pomalidomide are commonly used to treat multiple myeloma and other hematologic malignancies and are associated with increased risk of VTE. Current National Comprehensive Cancer Network (NCCN) guidelines (v.1.2016) on cancer-associated VTE utilize a risk assessment model based on a few small studies to guide VTE prophylaxis for multiple myeloma patients being treated with an IMiD (Palumbo, et al. Leukemia, 2008). Aspirin is recommended for lower risk patients while prophylactic-dose low molecular weight heparin or therapeutic warfarin are recommended for higher risk patients.
The use of direct oral anticoagulants (DOACs) for cancer-associated VTE is currently under investigation (Raskob, et al. Lancet Haematol, 2016). Little is known about their role in VTE prophylaxis in patients on IMiDs. The purpose of this study was to explore the use of DOACs in patients receiving IMiDs. We performed a retrospective chart review of all patients at the University of Virginia Health System who received an IMiD and who were taking either a DOAC or warfarin between January 1, 2010 and December 31, 2015. DOACs included dabigatran, rivaroxaban, and apixaban. IMiDs included lenalidomide, thalidomide, and pomalidomide. The primary endpoint was to assess the safety of DOACs as compared to warfarin, and the secondary endpoint was to assess their efficacy.
We collected baseline patient information, as well as diagnosis, IMiD, anticoagulant, antiplatelet agent and dose, duration of treatment, concurrent antineoplastic therapies, and thrombotic risk factors. We utilized the NCCN definitions of individual and myeloma-related thrombotic risk factors. Many patients had changes in their IMiD or anticoagulation. Thus, separate encounters were collected, where an encounter was the combination of an IMiD agent and dose, the anticoagulant, the antiplatelet agent (if any), and the thrombotic risk profile for that time period. Descriptive analyses were performed on all encounters in both groups. Rates of bleeding and thrombotic events were calculated.
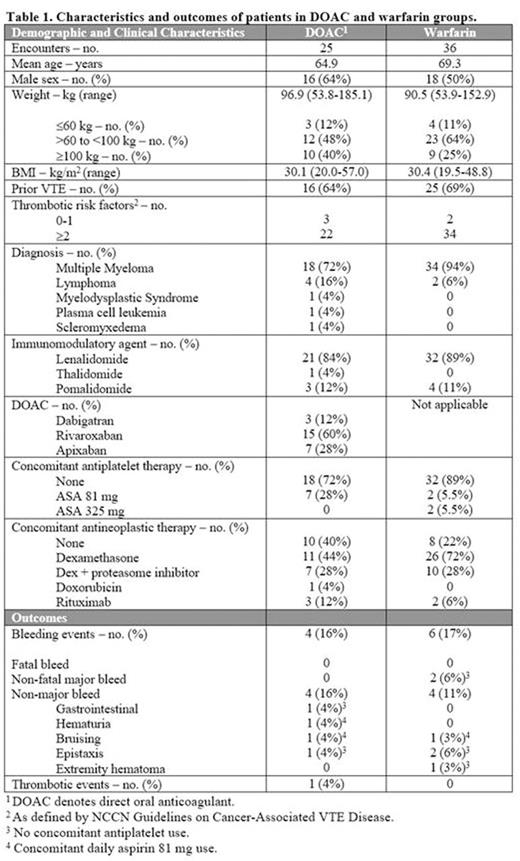
There were 21 discrete patients in the DOAC group and 16 in the warfarin group. Characteristics and outcomes are described in Table 1. There were four non-major bleeding events in the DOAC group; two of the four bleeding events occurred while on concomitant aspirin therapy. The patient with hematuria stopped anticoagulation and cystoscopy revealed bladder cancer. The other patients did not change or stop therapy. There were six bleeding events in the warfarin group: two were major and four were non-major. The two major events were gastrointestinal bleeding (GIB) and a subarachnoid hemorrhage (SAH). Neither event occurred while on concomitant antiplatelet therapy. The GIB required cessation of therapy, hospitalization, and transfusions. The INR at the time is unknown. The SAH was preceded by a fall while INR was in therapeutic range. Both major and two of the non-major bleeding events occurred in the same patient at different time points and accounted for most of the warfarin-related bleeding events. There was one thrombotic event in the DOAC group. The patient had a non-ST segment elevation myocardial infarction (NSTEMI) while on prophylactic-dose rivaroxaban. She was not on concomitant antiplatelet therapy. Arterial thrombotic events are not typically seen with IMiDs, and the NSTEMI was thought to be related to the recent initiation of carfilzomib. There were no thrombotic events in the warfarin group.
This was a retrospective, single-institution study assessing the safety and efficacy of DOACs as compared to warfarin in patients on IMiDs. In our study, all bleeding events in the DOAC group were non-major, while patients on warfarin experienced both major and non-major bleeds. Only one thrombotic event was recorded in the DOAC group. DOACs may represent an attractive alternative to warfarin for VTE prophylaxis in these patients, given no need for routine monitoring and fewer dietary restrictions. Prospective studies in this population are warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal