Abstract
Introduction: Andexanet alfa (andexanet) is a modified recombinant human factor Xa (FXa), developed to reverse the anticoagulation effects of both direct and indirect FXa inhibitors. For antithrombin III (ATIII)-dependent FXa inhibitors, such as enoxaparin, andexanet binds to the ATIII-enoxaparin complex with high affinity and reverses the inhibition of coagulation factors Xa and IIa. This study evaluated the ability of andexanet to reverse anticoagulation effects and blood loss due to the indirect FXa inhibitor, enoxaparin, in a rabbit liver laceration model.
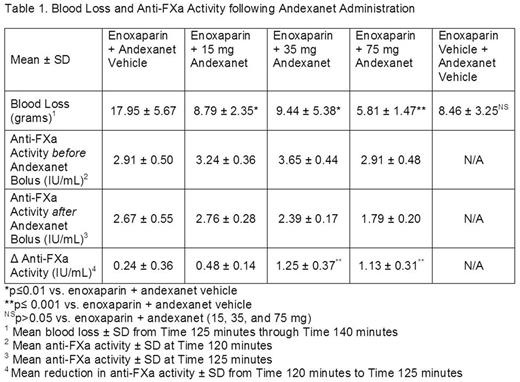
Methods: In a rabbit model of surgically-induced, acute hemorrhage (liver laceration model), rabbits were randomized to receive enoxaparin (8 mg/kg; subcutaneous bolus) or enoxaparin vehicle. This route of administration and dose of enoxaparin was based on the pharmacokinetic profile of enoxaparin and pharmacodynamic activity in the rabbit (2-fold increase in blood loss relative to non-anticoagulated rabbits). Andexanet (15, 35, or 75 mg per rabbit) or andexanet vehicle was administered to anesthetized rabbits as a 5-minute bolus, 120 minutes after enoxaparin injection (from Time 120 to Time 125 minutes), prior to livery injury. The five treatment groups were enoxaparin vehicle + andexanet vehicle, enoxaparin + andexanet vehicle, or enoxaparin + andexanet (3 dose levels). For liver laceration, a standardized injury (10 1-cm-long and 2- to 3-mm-deep incisions) was made into two liver lobes with 5 incisions in each lobe, and bleeding was allowed to progress for 15 minutes (from Time 125 to Time 140 minutes). Study efficacy endpoints included evaluations of blood loss in rabbits anticoagulated with enoxaparin, and anti-FXa activity in plasma.
Results: In rabbits anticoagulated with enoxaparin, andexanet significantly decreased blood loss 2-3 fold, relative to vehicle control (assessed at Time 140 minutes) at all doses of andexanet administered (15, 35, 75 mg per animal) (see Table 1). The mean blood loss in anticoagulated rabbits administered andexanet was similar to that in non-anticoagulated rabbits (enoxaparin vehicle + andexanet vehicle). In enoxaparin-anticoagulated rabbits, andexanet rapidly (5 minutes after the start of andexanet administration; Time 125 minutes) reduced mean anti-FXa activity, and the reduction was significant for the 35-mg and 75-mg doses of andexanet (see Table 1). Anti-FXa activity did not return to baseline levels for the duration of the study from Time 120 to Time 140 minutes. These results are consistent with previous studies in enoxaparin-anticoagulated rats where a similar decrease in anti-FXa activity was sufficient to significantly reduce blood loss. These findings with an indirect (ATIII-mediated) FXa inhibitor enoxaparin are distinct from those with a direct FXa inhibitor (e.g., rivaroxaban), which showed that larger reductions in anti-FXa activity were required for significant reductions in blood loss.
Conclusions: Administration of andexanet resulted in reversal of the anticoagulant effects of enoxaparin as well as restoration of hemostasis in enoxaparin-anticoagulated rabbits, suggesting it could be clinically valuable for the management of major bleeding associated with indirect FXa inhibitors. This study suggests a potential difference in the extent of reduction in anti-FXa activity required for significant reduction in blood loss associated with indirect vs. direct FXa inhibitors. Further studies are ongoing to better understand correlations between anti-FXa activity and blood loss following andexanet administration in enoxaparin-anticoagulated animals.
Pine:Portola Pharmaceuticals: Employment. Lu:Portola: Employment, Patents & Royalties. Canivel:Portola Pharmaceuticals: Employment. Pratikhya:Portola Pharmaceuticals: Employment. DeGuzman:Portola Pharmaceuticals: Employment. Karbarz:Portola Pharmaceuticals: Employment. Takeda:Portola Pharmaceuticals: Employment. Malinowski:Portola Pharmaceuticals: Employment. Leeds:Portola Pharmaceuticals: Employment, Equity Ownership, Patents & Royalties, Research Funding. Curnutte:Portola Pharmaceuticals: Employment, Equity Ownership, Patents & Royalties, Research Funding; 3-V Biosciences: Equity Ownership; Sea Lane Biotechnologies: Consultancy. Conley:Portola Pharmaceuticals: Employment, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal