Abstract
INTRODUCTION
Women with a history of venous thromboembolism (VTE) have a 2% to 10% absolute risk of developing pregnancy-associated recurrent VTE. Based on these figures, current guidelines recommend postpartum thromboprophylaxis in all pregnant women with a history of VTE. Women with a moderate or high risk of recurrent VTE (i.e. women with a history of VTE that is either unprovoked or associated with a hormonal or minor risk factor), should also receive thromboprophylaxis antepartum.
The optimal dose of low-molecular-weight heparin (LMWH) for thromboprophylaxis in pregnant women at moderate or high risk of recurrent VTE is currently unknown, as only two very small prospective trials, both with methodological limitations, have evaluated pharmacological prevention of recurrent VTE in this population. Therefore, the major international guidelines suggest the use of either a prophylactic or intermediate (half-therapeutic) dose of LMWH in this setting, without a preference of one dose over the other. The Highlow study is an investigator-initiated, multicenter, international, open-label, randomized controlled trial (RCT) that compares the efficacy and safety of a fixed dose of LMWH versus an intermediate dose of LMWH for the prevention of pregnancy-associated recurrent VTE (NCT 01828697; www.highlowstudy.org). The rationale and design features of the Highlow study were recently published [Bleker SM, Thromb Res, 2016]. Here, we present an interim report.
METHODS
In April 2013, we enrolled the first patient in the Highlow study. For the current report, we analyzed the enrolment rates and baseline characteristics of patients in the Highlow study between 1 April 2013 and 28 June 2016. Twelve centers recorded eligible women that did not give informed consent. Using the enrolment data from these centers, we analyzed the percentage of women giving informed consent, and reasons for not giving consent. All characteristics were summarized using descriptive statistics.
RESULTS
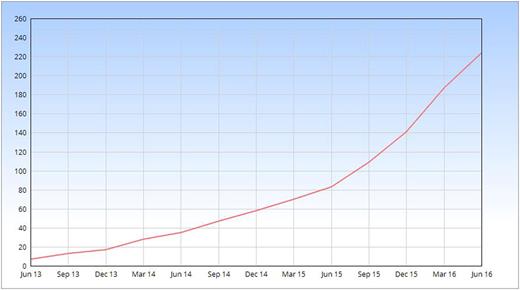
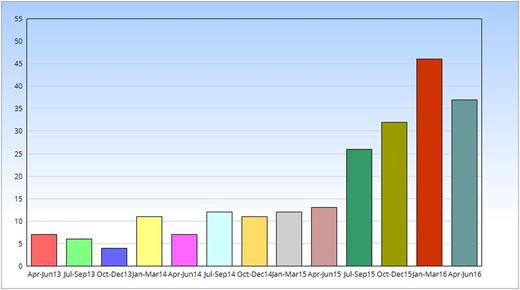
A total of 222 pregnant patients with a history of VTE that fitted the in- and exclusion criteria of the Highlow study were enrolled in 43 centers in 5 countries (Figure 1). The inclusion rate has been growing exponentially over time (Figure 2). In 12 centers that recorded eligible women not giving consent, 101 of 149 identified patients gave informed consent (68%). Patients who declined participation either did not give a specific reason (N=22; 46%), preferred a low dose of LMWH (N=20; 42%), preferred an intermediate dose (N=2; 4%), preferred a therapeutic dose (N=2; 4%), refused antepartum prophylaxis (N=1; 2%) or had been advised by another physician not to participate (N=1; 2%).
On 28 June 2016, baseline data in the CRF had been completed for 181 patients. Six patients participated twice. The mean age was 33 years ± 5.1, with a mean body mass index of 26.7 ± 5.9. The majority had a history of lower limb deep vein thrombosis (N=102; 56%) or pulmonary embolism (N=72, 40%). Of all previous VTE, the majority was hormone-related (N=97; 54%) or related to pregnancy (N=58; 32%). In total, 34 (19%) of prior VTE were unprovoked, and in only 19 cases (11%) a minor risk factor had been present. Thrombophilia screening had been performed previously in 101 patients (56%) and more than half of them (59 patients, 58%) had positive tests, especially for heterozygous factor V Leiden (N=39; 64%) and the heterozygous prothrombin 20210A mutation (N=13; 22%).
CONCLUSIONS
This report represents the largest number of pregnant women with a history of VTE participating in a RCT to date, and the future results are very likely to impact current clinical practice and consensus guidelines. Reassuringly, our enrolment rate shows that recruitment of pregnant women with a history of VTE is feasible. With the current enrolment rate we expect to include the last patient, reaching the event-driven sample size of approximately a 1000 patients, by the end of 2019. Hence, the outcome results can be expected in 2020.
SPONSORSHIP AND FINANCIAL SUPPORT
An unrestricted grant is provided by Aspen Pharma to the Academic Medical Center in Amsterdam (the Netherlands), who is the sponsor of the Highlow study. In France, where CHU de Saint Etienne is the sponsor, a grant was acquired from the French Ministry of Health (PHRC national 2014).
Buchmüller:French Ministry of Health: Research Funding. Chauleur:Sanofi: Honoraria; French Ministry of Health: Research Funding. Ní Áinle:Actelion UK: Research Funding. Verhamme:Boehringer Ingelheim: Honoraria, Research Funding; Bayer-Healthcare: Honoraria, Research Funding; Pfizer: Honoraria; Sanofi-Aventis: Honoraria, Research Funding; LeoPharma: Research Funding; Daiichi-Sankyo: Honoraria. Décousus:French Ministry of Health: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal