Abstract
Introduction
Venous thromboembolism is a frequent complication in cancer patients and results in a considerable morbidity and mortality. The underlying mechanisms leading to the increased thrombotic risk are yet poorly understood. We have previously shown that levels of extracellular vesicles (EV) are elevated in patients with colorectal cancer compared to healthy control individuals (Hron et al, Thromb Haemost 2007;97:119-123). EV originate from blood or endothelial cells, or from the underlying tumor itself. They may contribute to coagulation activation and propagation by exposing tissue factor and by providing a surface for the interaction of coagulation factors. In that study, the number of EV was also positively correlated with levels of D-dimer, a fibrin split product and marker of coagulation activation.
We hypothesize that number of EV and levels of D-dimer decline with decreasing tumor load during antineoplastic treatment. Therefore, the study aims at evaluating the long-term effect of chemotherapy on hemostatic system activation in patients with advanced colorectal cancer.
Methods
We conducted a pilot study including patients receiving chemotherapy because of advanced colorectal cancer. All chemotherapy regimens were based on 5-fluorouracilcombined with either oxaliplatin or irinotecan without or with an antibody (bevacizumab in 72%, cetuximab in 11%, and ramucirumab in 5% of patients, respectively). Patients were followed for 3 chemotherapy cycles. The study was approved by the local ethics committee, was conducted according to the Declaration of Helsinki and informed consent was obtained from all study patients. Venous blood was sampled at each cycle immediately before chemotherapy and was centrifuged at 2600 g for 15 minutes. The number of EV was assessed by flow cytometry using a FACSCalibur® flow cytometer with CellQuest™ software (Becton Dickinson) immediately after blood collection and centrifugation in fresh plasma. EV were defined by size (forward scatter, <1 µm) and annexin V binding. Tissue factor positive EV were characterized by an anti-CD142 antibody. Plasma was then frozen and stored at -80°C and was used for determination of markers of coagulation activation (D-dimer, prothrombin fragment f1.2) by commercially available ELISA kits.
All outcome variables were log-transformed due to skewed distributions. The paired t-test was used to compare baseline (before the 1st chemotherapy) levels with measurements obtained from the 2nd and 3rd blood sampling. In order to provide a clearer legibility, all data is presented in absolute numbers and all values are given as median (quartiles) if not otherwise stated.
Results
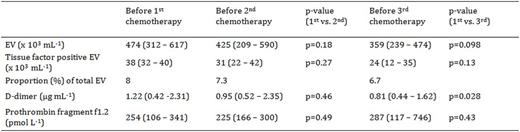
18 patients completed 3 cycles of chemotherapy. Their mean (± SD) age was 60.5 (± 12.2) years and 14 (78%) were men. None of the patients developed venous thromboembolism. Table 1 shows the levels of coagulation activation markers and the number of EV at baseline and before the 2nd and 3rd cycle of chemotherapy, respectively. D-dimer levels were 1.22 (0.42-2.31) µg mL-1 at baseline and significantly decreased over the course of treatment. D-dimer levels did not correlate with the number of EV either at baseline or at later time points. The number of EV decreased from 474 (312-617) x 103 mL-1 at baseline to 359 (239-474) x 103 mL-1 before the 3rd cycle. The proportion of tissue factor positive EV was small at baseline and throughout treatment. Levels of prothrombin fragment f1.2 did not change during treatment and did not correlate with number of EV at any time point.
Conclusions
In patients with advanced colorectal cancer chemotherapy attenuates coagulation activation as indicated by a decline of D-dimer levels and number of EV. These findings warrant further studies in a larger patient population and longer observation time.
Number of extracellular vesicles (EV) and markers of coagulation activation in plasma of colorectal cancer patients before and during chemotherapy
Number of extracellular vesicles (EV) and markers of coagulation activation in plasma of colorectal cancer patients before and during chemotherapy
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal