Abstract
Background: Heart failure affects more than 7 million people in the United States with over 250,000 patients in advanced stages with a dismal 1 year survival of less than 20%. For such patients, mechanical circulatory support (MCS) with continuous flow left ventricular assist devices (CF LVADs) and 1-year survival rates of >80%, has become a viable treatment option, either as a bridge to heart transplantation or as destination therapy. Although bleeding is a known major problem during CF LVAD support, a more devastating sequela is the rising incidence of thrombosis. The etiology of thrombosis remains uncertain. Hemolysis is associated with a prothrombotic state in patients with paroxysmal nocturnal hemoglobinuria and in individuals with hemoglobinopathies, and is a significant complication of CF LVADs. We hypothesized that early low level hemolysis, as assessed by LDH at the time of discharge, could be related to subsequent thrombosis and might serve as a predictive biomarker.
Methods: A retrospective single center review of all patients supported for at least 30 days by a Heart Mate (HM) II CF LVAD from January 2010 to July 2015 was conducted. All patients were on the same a priori antithrombotic regimen of aspirin 325 mg daily and warfarin therapy with a target INR of 2-3. Major demographics and LDH levels from the time of discharge of the HM II implant hospitalization were retrieved. Present, but low level hemolysis was defined by LDH levels of >400 U/L (normal range: 125-305 U/L). Cumulative survival free from a combined endpoint of confirmed device thrombosis and ischemic stroke (iCVA) was compared by using Kaplan Meier analysis. Device thrombosis was suspected by aberrant device parameters/malfunction and confirmed by device inspection after surgical exchange. Ischemic CVA was suspected based on neurologic symptoms and confirmed on Brain CT scan.
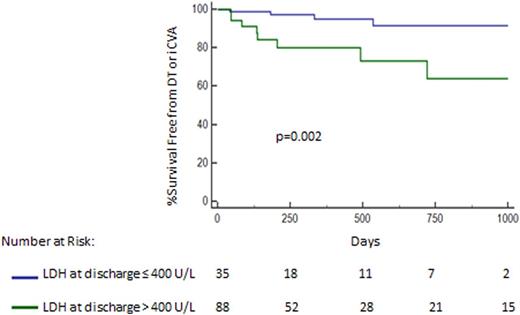
Results: Overall, 123 patients were supported for 428±347 days by the HM II. Twelve (9.7%) had a thrombotic event from either a confirmed device thrombus (n=6) or an iCVA (n=6). There were no significant differences in age (thrombosis: 48±13yrs, no thrombosis: 54±12yrs, p=0.1) and gender (thrombosis: 16% female, no thrombosis: 23% female, p=0.9). Patients with thrombosis had a higher LDH at discharge (504 ±307 U/L) in comparison to those without thrombosis (350 ±110 U/L, p<0.0001). Patients with a LDH >400 U/L (n=88) at discharge had an over 6-fold higher risk of subsequent device thrombosis or iCVA in comparison to those with LDH levels ≤400 U/L (n=35, HR: 6.2, 95% CI: 1.7-22.3, p=0.002, Figure 1). Despite salvage interventions after thrombosis such as device exchange or heart transplantation, there was a trend towards worse overall survival in patients with a discharge LDH >400 U/L (HR: 1.5, 95% CI: 0.92-2.5, p=0.07).
Conclusion: Low level hemolysis, as assessed by increased LDH levels at discharge during HM II support, is associated with thrombosis. Further studies to evaluate LDH as a predictive marker for thrombosis and investigations into the mechanisms of a hemolysis-induced pro-thrombotic state during CF LVAD support are warranted.
Goldstein:St. Judes Medical: Consultancy. Jorde:St. Judes Medical: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal