Abstract
Background: Noonan syndrome, characterized by dysmorphic facies, webbed neck, short stature, chest deformity, developmental delay and congenital heart defects, occurs as a result of mutations affecting the RAS-MAPK signaling pathway. A wide range of coagulation abnormalities are observed in these patients including: thrombocytopenia, platelet dysfunction and coagulation factor deficiencies. Screening for coagulation defects is recommended in childhood and especially prior to surgical procedures. Data available is limited and the extent of the hemostatic evaluation needed is not clearly defined. We carried out this study to outline the spectrum of coagulation abnormalities seen in patients with Noonan syndrome and their impact on surgical outcomes.
Methods: In this retrospective cohort study, the electronic medical record system was queried for patients with Noonan syndrome seen at Mayo Clinic between 2000 and 2016. A detailed chart review was undertaken.
Results
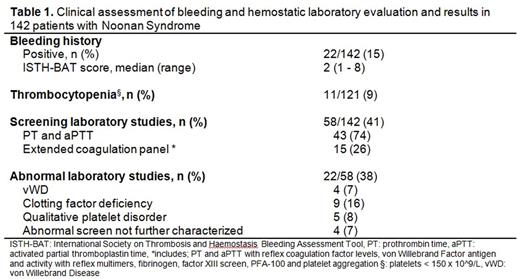
i) Phenotypic correlates: 142 patients (58% male) met our study criteria, median age 21 years (range 14 days to 68 years). The referral specialty for initial evaluation was: cardiology 69 (49%), endocrinology 10 (7%), urology 10 (7%), neurology 8 (6%), pulmonary 6 (4%), orthopedic surgery 3 (2%), gastroenterology 2 (1%), psychiatry 2 (1%) and other specialties 4 (3%). Ten patients (7%) were referred for developmental delay, 13 (9%) for dysmorphism and 4 (3%) after having a family member diagnosed with Noonan syndrome. One patient (1%) presented with hematochezia. Twenty-seven (52%) of the 52 patients screened with genetic testing had a Noonan-associated gene defect. Bleeding was reported by 22 patients (15%) with a median International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH-BAT) score of 2 (range 1-8). Of those tested, 11/121 (9%) patients had thrombocytopenia (platelets < 150 x 10^9/L). Screening coagulation tests were done in 58 (41%) patients, and included PT/aPTT in 43 (74%) and extended coagulation panels (including platelet function studies) in 15 (26%). Of the 22 patients with history of bleeding, 20 (90%) had screening coagulation testing. Twenty-two (38%) of patients screened had one or more coagulation laboratory abnormality. Ten (66%) patients with clinically suspected platelet dysfunction had normal platelet function analyzer (PFA)-100 testing and normal light transmission aggregometry, while 5 (33%) had decreased platelet aggregation with epinephrine. Four patients (7%) (including 2 siblings) had low vWF levels consistent with type 1 vWD, 9 (16%) had mild clotting factor deficiencies ( FIX- 3, FXII-2 and multiple clotting factors-5) and 4 (7%) had a prolonged baseline PT and/or aPTT without additional coagulation factor assessment (Table 1). Thirteen patients (9%) had both a bleeding phenotype and abnormal coagulation laboratory results.
ii) Surgical outcomes: A total of 274 surgical procedures were performed in 89 patients (63%). These included: cardiac 118 (43%), orthopedic 30 (11%), ENT 27 (10%), neurosurgical 26 (10%), abdominal 17 (6%), urologic 17 (6%), gynecologic 12 (4%) thoracic 8 (3%), ophthalmologic 7 (3%), plastic surgery 5 (2%), maxillofacial 4 (1%) and lymph node dissection 3 (1%). Platelets were utilized in 26 procedures (10%), fresh frozen plasma in 19 (7%) and cryoprecipitate in 7 (3%). Two procedures were performed in patients with type 1 vWD using vWF concentrates. Abnormal intra or postoperative bleeding was seen in 5/274 procedures (1.8%) performed in 4 patients. One patient had a known functional platelet disorder, one had normal PT and aPTT and two were not screened for coagulation defects. Bleeding was managed with transfusional supportive care in 3 (60%) surgical cases, while in 2 (40%) cases patients required re-intervention to achieve surgical hemostasis (bleeding from femoral access site and pacemaker pocket hematoma). There was no mortality related to the excess perioperative bleeding.
Conclusion: Varying hemostatic abnormalities are seen in patients with Noonan syndrome, ranging from thrombocytopenia to multiple coagulation factor deficiencies. Frank bleeding manifestations however are uncommon and when present are usually mild. Clinical evaluation focused on the bleeding history and basic coagulation screening allows for successful perioperative hemostatic support and good surgical outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal