Abstract
Background
Gastrointestinal bleeding (GI-B) is the most common major bleeding complication in oral anticoagulation. While direct oral anticoagulants (DOACs) have been shown to significantly decrease major bleeding, intracranial hemorrhage and fatal bleeding compared to vitamin k antagonists (VKA), there is conflicting evidence regarding the relative risk of GI-B. However, while bleeding rates are important, the exact localization of GI-B, the management, resource consumption and outcome of DOAC-associated GI-B could be even more important from a clinical perspective, but such data are currently lacking.
Methods
We analyzed all major GI-B events (defined as any GI-B that resulted in or occurred during hospitalization) documented in the prospective Dresden NOAC registry. Only patients with overt bleeding undergoing GI endoscopy were included in our analysis. Bleeding localization, lesion type, endoscopic treatment, consumption of blood and coagulation factor transfusion, length of stay and in-hospital mortality were compared with historic data from consecutive GI-B patients treated at our academic hospital between 2005 and 2010, before the market entry of DOACs to reduce the risk for selection bias. All patients underwent manual chart review to assure optimal data quality. Due to the nature of the datasets, only descriptive comparisons were performed.
Results
A total of 143 major GI-B episodes fulfilled our inclusion criteria (112 exposures to rivaroxaban, 18 to apixaban and 13 to dabigatran) and were compared against data from 1911 consecutive cases of GI-B in our historic cohort, of which 185 were exposed to VKA and 711 were exposed to antiplatelet drugs at the time of bleeding. Patient characteristics according to type of antithrombotic therapy are presented in table 1. GI-B locations could be identified in the upper GI tract in 63/143 DOAC cases (44.1%), in the lower GI tract in 60 (42.0%) and were in the middle GI-tract or not identified in the remaining 20 DOAC cases (14.0%). This pattern was different from bleeding patterns in patients with VKA or antiplatelet therapy (table 1), where most cases presented as upper GI-B and where, within lower GI-B, hemorrhoid bleeding was considerably less common compared to DOAC recipients. Within upper GI-B, 17/63 cases in DOAC recipients were due to an ulcer (27.0%), which is much lower than the ulcer rate in upper GI-B seen with VKA or antiplatelet drugs (table 1).
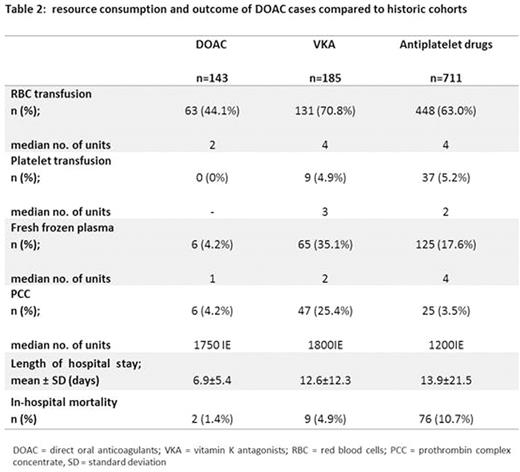
Transfusion requirements were lower in DOAC recipients compared to GI-B cases with VKA or antiplatelet drugs (table 2). Similarly, length of hospital stay was shorter in cases with DOAC therapy compared to VKA or antiplatelet drug recipients.
Finally, patients with GI-B during DOAC therapy demonstrated an in-hospital mortality of only 1.4%, which compared favorably against the in-hospital mortality of GI-B cases exposed to VKA (4.9%) or antiplatelet therapy (10.7%; table 2).
Of the 17 DOAC patients with upper GI bleeding due to an ulcer, none died in hospital, whereas ulcer-associated upper GI bleeding was associated with an in-hospital mortality of 11.3% in VKA and 13.5% in antiplatelet drug recipients. In contrast, in-hospital mortality was 0% for hemorrhoid bleeding with DOAC, VKA and antiplatelet therapy.
The two fatal GI-bleedings in the DOAC cohort occurred in 84 and 95 year old female patients and manifested as diffuse mucosal bleeding in the upper GI tract.
Conclusions
Our data indicate that, within GI-B, different bleeding localizations have different outcomes. Patients with GI-B during DOAC therapy have more often lower GI bleed compared to bleeding patients with VKA or antiplatelet drugs, which is driven by a large proportion of hemorrhoid bleeding. In contrast, upper GI-B is much less frequent and less common related to ulcers. DOAC-associated GI-B resulted in comparatively low resource consumption, shorter hospitalization and a low in-hospital mortality compared to GI-B historically seen with VKA or antiplatelet agents. The difference in resource consumption and mortality is likely due to a shift in bleeding patterns, together with the short half- life of DOACs.
A focus on GI-B rates with different antithrombotic therapies alone may be misleading, since bleeding localization, resource consumption and outcome may vary considerably. These findings need to be confirmed in larger cohorts, ideally from randomized controlled trials, to allow for statistical comparisons.
Pannach:Bayer: Honoraria; Pfizer: Honoraria. Marten:Daichii Sankyo: Honoraria; Bayer: Honoraria. Beyer-Westendorf:Daichii Sankyo: Consultancy, Honoraria, Research Funding; Boehringer Ingelheim: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; LEO: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal