Abstract
Background: von Willebrand disease (vWD) is the most common inherited bleeding disorder characterized by quantitative or qualitative defect of von Willebrand factor (vWF) production. Patients with vWD are at high risk of bleeding during surgery. We aimed to evaluate the efficacy and safety of factor VIII (FVIII)/vWF concentrates and DDAVP for bleeding prevention in vWD patients who undergo surgery or an invasive procedure.
Methods:The electronic searches were performed in MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials databases. The articles published from inception to January 2016 were eligible for inclusion in this review. Studies will be included if they are observational studies or clinical trials of pediatric or adult patients who were laboratory confirmed vWD (type 1 or type 2 or type 3). We excluded case report or case series with less than 5 patients, acquired vWD and incomplete outcome reports. The primary outcome was the efficacy of hemostatic control as defined by studies. We calculated pooled incidence of the patient who achieve good or excellent hemostatic control with corresponding 95% confidence interval using single proportion meta-analysis. The secondary outcomes were adverse outcomes from FVIII/vWF and DDAVP.
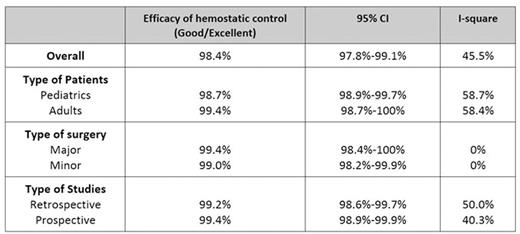
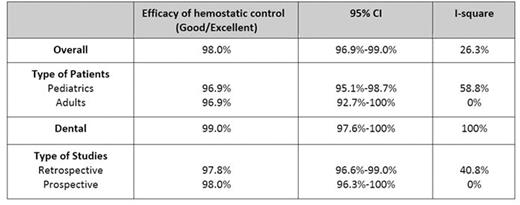
Results: After screening, there were 54 studies included in the meta-analysis. Forty-five studies investigated the efficacy of vWF/FVIII. Pooled analysis revealed that 98.4% (95% CI; 97.8-99.1%, I2=45.5%) of FVIII/vWF treated patients achieved excellent or good hemostasis. Subgroup analysis according to type of surgery revealed that excellent or good hemostasis was 99.4% (95%CI; 98.4-100%, I2=0%) in major surgery and 99.0% (95%CI; 98.2-99.9%, I2=0%) in minor surgery, Table 1. Adverse effects were observed in 0.2% (95% CI; 0-0.4, I2=85.1%) patients who received FVIII/vWF. The reported adverse effects from FVIII/vWF concentrates included phlebitis, shortness of breath, chest tightness, urticaria, pruritus, chill, malaise, fever, paresthesia, transient elevation of liver function test, nausea, vomiting, dizziness, headache and parvovirus B 19 seroconversion. Thromboembolism occurred in 0.17% of patients who received vWF/FIII. There were 26 studies investigating DDAVP during surgery. Hemostasis was excellent or good in 98.0% (95%CI; 97.0-99.0%, I2=26.3%. In dental procedure, 99.0% of patients receiving DDAVP (95%CI; 97.6-100%, I2=0%) achieved excellent or good hemostasis. Subgroup analysis showed that good/excellent hemostasis was found in 96.9% of pediatric patients (95%CI; 95.1-98.7%, I2=58.8%) and 96.9% (95%CI; 92.7-100%, I2= 0%) in adult patients, Table 2. Adverse effects from DDAVP were reported in 1.2% (95%CI; 0.4-2.1%, I2=95%). Hyponatremia of any level was observed in 16.1% (95%CI; 8.9-22.4%, I2=94.1%). The other reported adverse effects were headache, fatigue, rash, seizure (from hyponatremia), flushing, dizziness, peripheral edema, nausea and vomiting. No thromboembolic event was found in DDAVP treated patients.
Conclusions: Both FVIII/vWF concentrates and DDAVP are effective and safe for bleeding prevention during surgery or an invasive procedure in vWD patients.
Efficacy of hemostatic control in patients receiving vWF: Factor VIII concentrate
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal