Abstract
Background: Peg-filgrastim (peg-G) is widely used in oncology to accelerate hematopoietic recovery after cancer chemotherapy. It is used occasionally for the treatment of severe chronic neutropenia (SCN). Because of frequent questions from clinicians, we reviewed the use of peg-G, its effectiveness and associated adverse effects for SCN patients followed prospectively through the Seattle office of the Severe Chronic Neutropenia International Registry (SCNIR).
Methods: The SCNIR is a long-term observational study of children and adults with congenital, cyclic, idiopathic and autoimmune neutropenia. They are enrolled according to a protocol requiring a minimum of 3 absolute neutrophil counts (ANCs) <0.5 x 109/L over at least a 3 month period. Patients are treated according to the practices of individual physicians; treatments are not prescribed by the study protocol or the SCNIR. Patients and their physicians regularly report treatments for neutropenia including drug doses, schedules, blood counts, infections and associated adverse events. SCNIR-Seattle includes patients in North and South America and Australia. Patients are followed through annual report forms and notes whenever serious illness or adverse events occur.
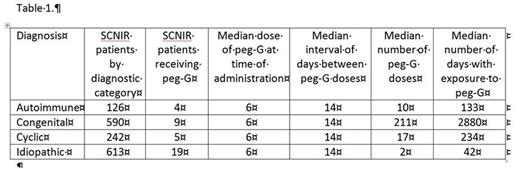
Results: Peg-G has been administered to 37 of 1571 patients enrolled in the SCNIR-Seattle: 12 children (age <18 years) and 25 adults (age >18 years). Common reasons for peg-G treatment were: convenience, patient dislike of injections, or poor response to filgrastim. Table 1 summarizes peg-G exposures:
All of the patients responded to peg-G with an increase in ANC over their baselines, but detailed pharmacodynamics to show the patterns of the rise and fall of the counts were not available. As shown in Table 1, the duration of treatment was relatively short for three groups: idiopathic, autoimmune and cyclic patients. In these patients, bone pain is a common adverse effect of the fixed doses of peg-G usually administered. Sweet syndrome was reported for only 1 patient with idiopathic neutropenia.
The majority of the reported use of peg-G is for patients with congenital neutropenia who are relatively refractory to G-CSF; 4 of 9 peg-G treated congenital neutropenia patients had been previously treated with G-CSF >10 mcg/kg/day. In the congenital subgroup, adverse events included splenomegaly (8), hepatomegaly (4) and AML (2). Following are brief descriptions of the cases of MDS/AML:
Patient #1: A 38 year old female initially diagnosed with SCN in 1989 at age 11 (ELANE mutation C223ter) and started G-CSF at 11.5 mcg/kilograms per day. Because of a poor response, G-CSF was increased to 24 mcg/kg/day. After 15 years she was switched to peg-G (3mg/injection) approximately every 10 days for the next 11 years. At age 37 she had increased blasts in the blood and marrow, leading to the diagnosis of AML and HSCT.
Patient #2: A 25 year old female (unrelated to patient #1) was initially diagnosed with SCN at 4.5 months of age (also ELANE mutation C223ter). At age 5 months she started G-CSF (5 mcg/kg/day increased soon to 20 mcg/kg/day). After 12 years, she was switched to peg-G (6 mg/injection) because of poor neutrophil response, and she received peg-G every 14 days for the next 12 years. She became pregnant (previously 3 pregnancies: 2 terminations and one live birth). Early in the pregnancy she appeared to be losing her response to peg-G. Her gums were infiltrated by immature myeloid cells and there were excess blasts in her bone marrow leading to the diagnosis of AML. With intensive care, she delivered a male infant at 30 weeks via C-section and then underwent an HSCT five weeks thereafter.
Conclusions: Patients with severe chronic neutropenia respond to treatment with peg-G but it is not easy to determine the proper dose and dosing interval. Bone pain is a common early adverse effect and Sweet syndrome may occur. In patients with congenital neutropenia who are refractory to G-CSF prolonged responses have been achieved lasting for many years with regular injections of peg-G at approximately 14 day intervals. However, 2/9 refractory congenital neutropenia patients developed AML after 11 and 12 years, respectively. Based on this experience, we recommend that peg-G should only be used in congenital neutropenia patients who are refractory to G-CSF (>20 mcg/kg/day) while searching for a donor for HSCT.
Boxer:Amgen: Equity Ownership. Dale:Amgen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal