Abstract
Background: Mutations in ELANE are the most common cause for both cyclic and severe congenital neutropenia. We previously reported that some mutations, e.g., G214R and C151Y, are associated with more severe outcomes. (Makaryan, Zeidler, et al; Curr Op Hematol. 2015;22:3-11) We also found that termination and frameshift mutations are also associated with a high risk of MDS/AML, death from infection or need for transplantation. To aid in clinical care of SCN patients, we compared the clinical characteristics of patients with termination and frameshift who have or have not developed MDS/AML.
Methods: We reviewed clinical records for 403 patients with severe chronic neutropenia and mutations in ELANE. We identified 44 patients with termination or frameshift mutations, both categories predicted to lead to synthesis of mutant neutrophil elastase that is missing a number of terminal amino acids and causing distortion of the three dimensional structure of the protein.
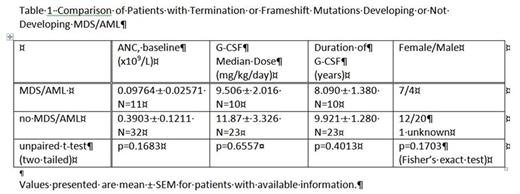
Results:There are currently 11 cases of MDS/AML in the 44 patients with termination or frameshift mutations. Table 1 compares clinical characteristics of these two groups.
There was no significant relationship of baseline ANC values, G-CSF dose or duration of G-CSF treatment. There appeared to be a higher risk of AML for the female patients, but the difference was also not significant.
The leukemia cases occurred with mutations in exon 3 (1), exon 4 (1) and exon 5 (9). The mutations in the group without MDS/AML were in exon 4 (8) and exon 5 (25). To date, there have been six distinct frameshift mutations associated with MDS/AML. There have been 3 distinct termination mutations associated with MDS/AML; three unrelated patients have the identical mutation C223ter. All of the patients in the SCNIR database who have this specific mutation have developed MDS/AML.
Conclusion: Frameshift and termination mutations appear to convey a high risk of MDS/AML in patients with severe chronic neutropenia. We believe patients with these mutations should be on a "watch list" with particular attention to surveillance and regular bone marrow examinations. Mutation C223ter, together with G214R and C151Y, appear to be particularly high risk. We recommend that all patients with these mutations be considered for early HSCT.
Dale:Amgen: Consultancy, Honoraria, Research Funding. Boxer:Amgen: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal