Abstract
Introduction: Chronic kidney disease (CKD) is common in patients with sickle cell disease (SCD). Despite current practice and recent NHLBI guidelines, which recommend screening and treatment for albuminuria, the progression of CKD in SCD and factors associated with such progression remain poorly defined. The purpose of this study was to evaluate the prevalence of CKD and its rate of progression in adult SCD patients. We also evaluated the laboratory and clinical factors associated with CKD progression.
Methods: We conducted a retrospective study of patients seen between July 2004 and December 2013 at an adult Sickle Cell Clinic. Patients had confirmed diagnoses of SCD, were at least 18 years old, and were in the non-crisis state at the time of evaluation. Patients were excluded for histories of HIV, hepatitis B and C, and systemic lupuserythematosus. Clinical and laboratory variables were obtained from medical records. Estimated glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation.Presence of CKD was assessed by using a modification of the Kidney Disease Improving Global Outcomes clinical practice guidelines that incorporateseGFR and levels of albuminuria. CKD was defined aseGFR<90 or presence of proteinuria (at least 1+ on dipstick urinalysis). A linear mixed effects model was used to analyze the rate ofeGFR decline with a random intercept and fixed time effect for each subject. Linear mixed effect models were also used to identify risk factors for decline ofeGFR - one utilizing laboratory values and the other utilizing demographic and clinical characteristics.
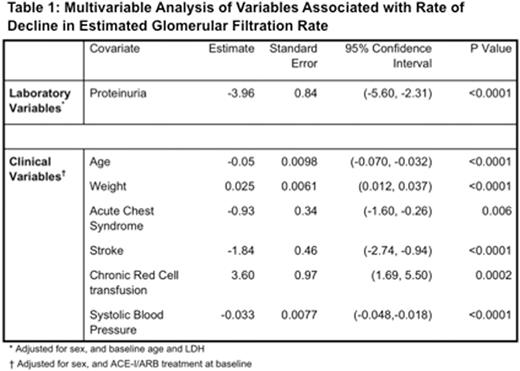
Results: Four hundred and twenty six patients with SCD (SS = 268, SC = 98, Sb0 = 22, Sb+ = 29, SE = 3, SD = 2, SHPFH = 4), median age of 29.5 years (IQR: 20 - 41 years), were evaluated. CKD at baseline was observed in 92 patients (21.6 %). The rate of decline in eGFR over time was 2.1 mL/min per 1.73 m2 per year (SE: 0.11, p < 0.0001) (Figure 1). Baseline laboratory factors that were significantly associated with decline ineGFR inunivariate analyses were hemoglobin, lactate dehydrogenase, indirect bilirubin, ferritin, hematuria, urine specific gravity, and proteinuria (at least 1+ on dipstick urinalysis). Clinical variables significantly associated witheGFR decline inunivariate analyses were weight, history of acute chest syndrome,history of stroke, chronic transfusion, systolic blood pressure, diastolic blood pressure, and use of ACE inhibitors/angiotensin receptor blockers (ACE-I/ARB). Multivariable analyses showed that the rate ofeGFR decline was dependent on the status of having proteinuria (estimate: -3.96, p < 0.0001), age (estimate: -0.05, p<0.0001), weight (estimate: 0.025, p<0.0001), systolic blood pressure (estimate: -0.033, p<0.0001), stroke (estimate: -1.84, p<0.0001), acute chest syndrome (estimate: -0.93, p=0.006), and chronic transfusions (estimate: 3.60, p=0.0002) (Table 1).
Conclusion: eGFR declined at a rate of 2.1 mL/min per 1.73 m2 per year in adult patients with SCD. Proteinuria, age, acute chest syndrome, stroke, and higher systolic blood pressure were associated with an increased rate of decline in eGFR. However, heavier weight and chronic red cell transfusions were associated with less severe eGFR decline. Better understanding of these relationships and their pathophysiology is needed so that we can modify identified risk factors and attenuate the loss of kidney function in SCD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal