Abstract
Background: Pain is the leading cause of hospitalization and pediatric emergency department (ED) visits for children with sickle cell disease (SCD). The National Heart, Lung and Blood Institute (NHLBI) recommends an initial dose of parenteral opioids within 30 minutes (min) of triage for moderate-severe vaso-occlusive pain episodes (VOEs) in the acute care setting. Delays in pain management for children with SCD commonly occur across EDs despite these guidelines. Intranasal fentanyl (INF) provides rapid and powerful parenteral analgesia, with an onset of action of 5-10 min, and peak in 30 min. INF is a safe and effective method of pain management for children in the ED and other clinical settings.
Objective:To assess the use and impact of INF and other common therapies for the treatment of children with SCD and VOE on discharge rates in EDs across the United States and Canada.
Methods: A retrospective cohort study was performed evaluating practices in 20 consecutive charts per site from 20 high-volume EDs (n=400 charts total) including 14 Pediatric Emergency Care Applied Research Network (PECARN) sites, assessing children age 3-21 years with SCD/VOE requiring parenteral opioids. Variables evaluated for association with discharge included age, gender, triage level, time from triage/room placement (whichever came first) to 1st parenteral opioid use (intravenous (IV) or intranasal), INF use, dose of total parenteral opioid (TPO) equivalents (mg/kg/hr), ketorolac use, oral opioids, any use of IV fluids (bolus and/or maintenance), ED shift presentation (AM, PM or overnight) and site. Multiple logistic regression was performed using significant variables identified on univariate analysis. Regression analyses were restricted to the 15 sites that had INF available in their ED (n=294 subjects).
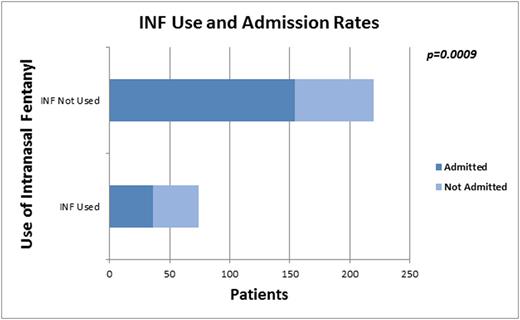
Results: Mean age of the 400 children was 14±5 years; 54% were female; 92% had HbSS. Admission rate was 67%. All received parenteral opioids; 84% IV fluids, 66% ketorolac, and 26% oral opioids. INF was available at 15/20 sites and used at 10/20 sites; 19% (75/400) of all children received INF, 25% when restricted to the 15 sites with INF available. INF was used in 60% of patients receiving parenteral opioids within 30 min of triage/room placement at sites with INF available. Median time to 1st parenteral opioid in children receiving INF was 25 (26, 37) vs. 64 (38, 102) min (p<.0001) for those who received only IV opioids. The admission rate was 49% vs. 70%, p=.0009 (Fig 1) for those treated vs not treated with INF. Variables associated positively with discharge included: shorter time from triage to 1st parenteral opioid (p<0.05), INF use (p<.001), oral opioid use (p<.0001), younger age (p=0.03), and site (p<.0001), while increased TPO equivalents (p=0.05), and IV fluid use (p=.01) were negatively associated with discharge. On multiple logistic regression analysis, INF use, TPO, use of IV fluids and site remained in the model. The Odds Ratio (OR) and 95% Confidence Interval for INF use and ED discharge was 2.99[1.14, 7.82], p=.03 for those who received INF compared to those who did not. Time from triage to 1stopioid dose did not affect admission risk in multivariate analysis.
Conclusions:It is notable that the odds of being discharged from the ED are nearly 3 times higher for patients who received INF compared to those who did not. Contrary to common belief, rapid delivery of first parenteral analgesia, a focus of the 2014 NHLBI guidelines for treatment of SCD/VOE, did not affect risk of admission in our cohort when controlling for other factors, although potential benefits related to pain relief and patient satisfaction were not evaluated. Variations in practice across the network and variables associated with ED discharge rate require further investigation. Causality of INF impact on discharge rates can't be shown without further study, however the rapid onset-of-action and ease of delivery without IV access offered by INF makes it an ideal initial parenteral analgesic in the treatment of children with SCD and pain in the acute care setting.
Morris:MAST: Research Funding; Pfizer: Consultancy; Calithera: Consultancy; Nourish Life: Patents & Royalties: I am the inventor of IP owned by UCSF-Benioff Children's Hospital that is licensed to NL; Endeavor: Consultancy; Nestle: Honoraria. Dampier:Eli Lilly and Company: Consultancy, Research Funding. Hsu:Gerson Lehman Group: Consultancy; Centers for Medicare and Medicaid Innovation: Research Funding; Astra Zeneca: Consultancy, Research Funding; Sancilio: Research Funding; Purdue Pharma: Research Funding; EMMI Solutions: Consultancy; Mast Therapeutics: Research Funding; Hilton Publishing: Consultancy, Research Funding; Eli Lilly: Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal