Abstract
Iron toxicity is the main risk factor for morbidity and mortality in patients with transfusion-dependent thalassemia. Current practice is to start chelation therapy only after 10-20 transfusions, or when the serum ferritin (SF) level rises above 1,000 μg/L. This delay is aimed at minimizing the risk of chelation toxicity that was observed with the use of deferoxamine in children with low iron stores. Deferiprone has lower affinity for iron than deferoxamine and data from clinical trials on its use in patients without systemic iron overload indicate a safety profile for its use in those conditions. The current trial was designed to evaluate the safety of the early use of low-dose deferiprone in newly diagnosed pediatric thalassemia and to evaluate if it can postpone iron overload.
Sixty-four children recently diagnosed with thalassemia major who had begun receiving blood transfusions every 3-4 weeks to keep pre-transfusion Hb above 10 gm/dl, had not yet started iron chelation therapy and had SF ≥ 400 μg/L or transferrin saturation (TSAT) ≥ 70% or labile plasma iron (LPI) ≥ 0.2 µM were randomized to start deferiprone (DFP) at a dose normally considered to be sub-therapeutic (50 mg/kg/day) or no chelation (NC). Age at 1st transfusion was 8.1 ± 1.7 for DFP-treated and 8.1 ± 1.6 months for NC children. The percentage of patients with LPI ≥ 0.6 µM, SF ≥ 1000 μg/L or TSAT ≥ 70% in each study arm was assessed at 6 months and 12 months. Patients with confirmed SF ≥1000 ng/mL were withdrawn from the study and placed on a standard chelation regimen.
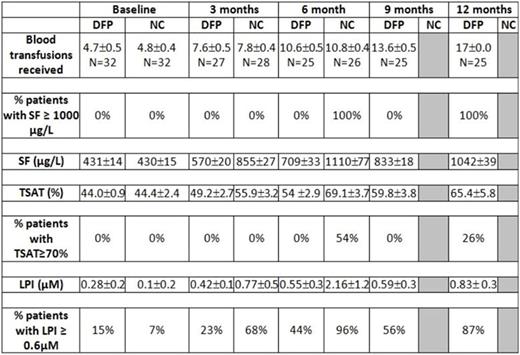
Results. Two patients (DFP) were lost to follow after baseline measurements, 1 patient (NC) withdrew consent at baseline, and 10 patients (5 DFP, 5 NC) have yet to complete all follow up visits. All NC patients had been removed from the trial prior to completing 7 months of follow-up 12 due to confirmed SF ≥ 1000 μg/L. Mean ± SD time of follow up was 10.4± 4.9 and 5.9 ± 2.5 months for DFP and NC, respectively. Most common adverse events in patients on DFP versus NC were diarrhoea (19% vs 13%, p= 0.73), vomiting (13% vs 13%, p=1.00), abdominal colic (13% vs 13%), increased liver enzymes (6% vs 3%, p=1.00) and neutropenia (neutrophil count between 1,000-1,500 x 109/L) (6% vs 6%). All adverse events were mild in severity and did not require interruption of DFP use. There were no cases of agranulocytosis or of moderate neutropenia, no arthralgia and no serious infections in DFP-treated patients. Preliminary efficacy results are presented in the table. DFP therapy was associated with a significant reduction in the rate of iron accumulation as measured by SF (P<0.0001) (Figure 1), LPI (P<0.001) (Figure 2) and TSAT (P<0.001). LPI ≥ 0.6 µM appeared as early as after 5 transfusions in NC children and was delayed to at least 10 transfusions with DFP therapy. TSAT ≥ 70% appeared after 10 transfusions in NC children and was delayed to at least 17 transfusions with DFP therapy.
The results of this study show that LPI and TSAT may reach values ≥ 0.6 µM and ≥ 70%, respectively, after 5 -10 transfusions in children with TM and all NC children had SF ≥ 1000 μg/L after 8-9 transfusions. A sub-therapeutic dose regimen of deferiprone for a mean of 10 months in children with TM and low iron overload was not associated with safety concerns and able to significantly reduce the rate of iron accumulation as measured by SF and the appearance of high levels of LPI and of TSAT.
Berdoukas:ApoPharma Inc: Consultancy. Tricta:ApoPharma Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal