Abstract
INTRODUCTION: Herein we present safety and efficacy results of the first American prospective study of intravenous (IV) iron (Fe) for oral (PO) Fe intolerant patients with iron deficient anemia (IDA) in the 2nd or 3rdtrimester (IND 114696, ClinTrials.gov NCT 02038023). Anemia affects up to 42% of gravidas. Neonatal Fe deficiency (ID) is associated with low birth weight, delayed growth, development and statistically significant increment in cognitive and behavioral abnormalities up to 10 years after Fe repletion. Although PO Fe is simple to use, inexpensive, up to 70% of gravidas to whom it is prescribed report significant gastrointestinal (GI) side effects. The availability of formulations convenient to administer in a brief single setting with favorable side-effect profiles may help decrease rates of anemia in pregnancy and improve hemoglobin (Hb) responses, maternal and fetal outcomes in women intolerant of, or unresponsive to, PO Fe.
METHODS: We treated 74 women with a Hb concentration <10.5 g/dl during the 2nd or <11.0 g/dl in the 3rd, with laboratory evidence of IDA (serum ferritin <20 ng/ml and/or % transferrin saturation (TSAT) <16%). A questionnaire with historical questions of metallic taste, gastric cramping, worsening or onset of constipation was administered to document PO Fe intolerance before enrollment. Women diagnosed during the 1st trimester were eligible for inclusion, however due to a lack of published 1st trimester safety, IV Fe infusion was delayed until beginning of 2nd. All received 1,000 mg LMWID (INFeD, Allergan, CA) diluted in 250 ml of normal saline. 15 minutes after a 25 mg test dose administered over 2 minutes, the remainder was infused over the balance of 1 hour. Subjects were observed for 1 hour for signs of acute hypersensitivity and called at 24, 48 hours and 7 days later to assess delayed reactions. Prior to treatment a visual linear analog scale analysis (LASA) for energy and activity was completed. 4 weeks post- infusion or post-partum depending on confinement, a follow-up visit for Hb levels, Fe parameters (TSAT and serum ferritin) and repeat LASA were performed. Paired T-test was used to assess changes in Hb and Fe parameters, and the Wilcoxin Signed-Rank Test for LASA changes. Following accrual of the final subject 58/73 were contacted and questioned for abnormalities in growth or development of their babies.
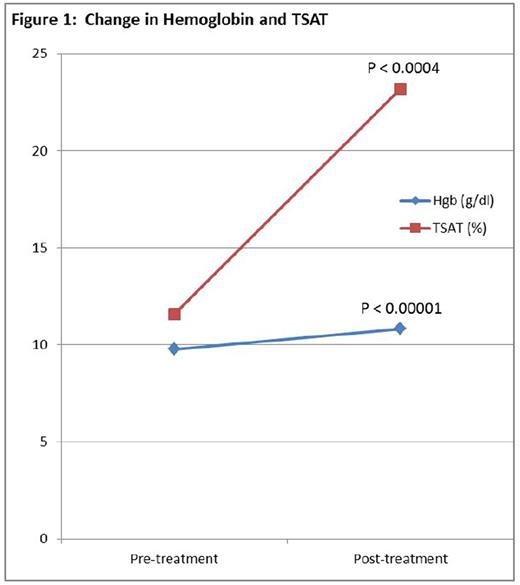
RESULTS: 73 of 74 enrolled subjects were treated. 1 experienced a minor infusion reaction (facial flushing) and requested treatment on another day. 72 hours later, labor commenced. 2 months post-partum IV Fe was given off study. Follow-up data were available in 58/73 subjects. The mean pre and post treatment Hb concentrations were 9.8 (SD 0.74) and 10.8 (SD 1.30) g/dl respectively (P<0.00001). The mean pre and post TSATs were 11.6 (SD 8.72) and 23.2 and the mean pre and post serum ferritins were 14.2 (SD 22.96) and 129.4 (SD 89.48) ng/mL. respectively (P<0.000001 for both). Data for 58 infants was available; one was low on its growth charts for 11 months after which the curve normalized. The remaining 57, ages 6 weeks to 3 years, were all normal with no evidence of any developmental or growth lag. None were diagnosed with IDA. 6 (8%) experienced minor self-limited infusion reactions consisting of facial flushing or myalgias of the chest or back. All resolved without therapy. 5 of 6 received the planned therapy. 2 developed mild myalgias and headache 24 hours after the infusion, with complete resolution at 48 hours. 85% reported improvements on repeat LASA (P<0.01).
DISCUSSION: The high prevalence of IDA in gravidas and its association with adverse maternal and infant outcomes, with a reported 2-fold increment of preterm labor and 3-fold increase in low birth weight, is concerning. PO Fe, the current standard, is fraught with GI toxicity and poor adherence in up to 70% to whom it is prescribed. Our prospective data corroborate a litany of studies, that IV Fe is a safe, effective and convenient method of Fe repletion. No significant adverse events have been reported. 96% had significant improvements in Hb, and 85% energy and activity. No adverse fetal outcomes were observed. The improved side effect profile of IV formulations allowing Fe repletion in a single, brief visit supports moving IV iron closer to frontline. Compared to PO Fe, IV Fe has fewer side effects and nearly always effective. Our data and those of others call for large prospective studies of IV vs. PO Fe for therapy of maternal IDA.
Auerbach:AMAG Pharma: Consultancy, Research Funding; Pharmacosmos: Consultancy, Research Funding; American Regent/Luitpold: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal