Abstract
Background: Patients undergoing hematopoietic stem cell transplantation (HSCT) are routinely screened for psychological and social risk factors prior to transplantation to determine eligibility and optimize support. Although patients with psychosocial risk factors have been shown to have a higher rate of depression and anxiety, the relationship between psychosocial risk and clinical outcomes in HSCT is not well established. We hypothesized that psychosocially vulnerable patients would be at a higher risk for adverse clinical outcomes including hospital readmission and increased length of stay (LOS) following HSCT.
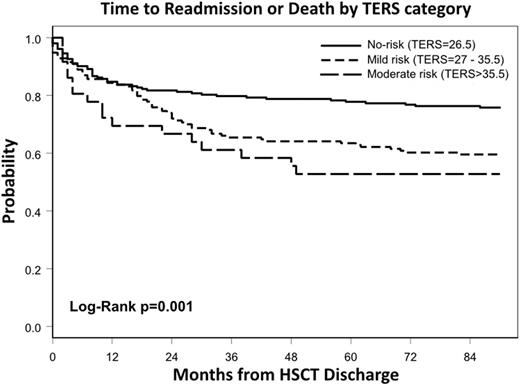
Methods: We performed a retrospective observational study at a single center of 395 consecutive HSCT patients who underwent pre-transplant psychosocial screening using the Transplant Evaluation Rating Scale (TERS). The TERS is a validated screening tool utilized to identify patients who are psychosocially vulnerable (κ=0.89-0.98, α=0.76, p=<0.001). TERS scoring is based on an aggregate of 10 different factors (Axis I diagnosis, Axis II diagnosis, substance abuse, compliance, health behaviors, family/social support, coping history, disease coping, affect quality, and mental status). The primary endpoint was time to readmission or death (TTRD) and was calculated as time from transplant discharge to readmission or death, censoring patients without an event at last follow-up or 90 days post-transplant. Estimates for TTRD were obtained using the Kaplan-Meier method and compared by the log-rank test. Multivariable regression analyses were performed to determine hazard ratios (HR) of TERS adjusting for other important covariates.
Results: Patients underwent autologous (n=218, 55%) and allogeneic (n=177, 45%) HSCT for acute leukemia (n=140, 35%), multiple myeloma (n=134, 34%), lymphoma (n=93, 24%) and other indications (n=28, 7%). Patients were classified according to psychosocial risk as no-risk (TERS=26.5, 52%) vs. at-risk (TERS>26.5, 48%), with at-risk patients subdivided into mild risk (TERS=27-35.5, 39%) and moderate risk (TERS>35.5, 9%). Psychiatric conditions (24%), poor health behaviors (16%), and poor coping history (13%) were the most common identified risk factors while substance abuse (7%) and non-compliance (2%) were less frequent. Characteristics were similar among TERS groups with respect to race, disease, remission status, type of HSCT, and graft source. Patients with higher TERS scores tended to be younger (no-risk [median age = 58 years] vs. moderate risk [median age = 50.5 years], p=0.04). In multivariable analysis controlling for comorbidity index and disease type, at-risk patients were significantly more likely to be readmitted within 90 days (mild risk HR=1.53 (95% CI [1.04-2.26], p=0.03), moderate risk HR=1.88 (95% CI [1.08-3.29], p=0.03)). This association held across diseases and HSCT type when examined separately. Additionally, in the multiple myeloma cohort, mild risk patients tended to have longer LOS (mean LOS = 19.06 days) than no-risk patients (mean LOS = 16.78 days, p=0.005). Overall survival did not differ significantly among groups.
Conclusion: All patients who are identified pre-transplant to have any degree of psychosocial vulnerability are at higher risk for hospital readmission following HSCT and certain groups are at risk for longer length of stay. Hospital readmission in HSCT patients is associated with poor overall survival, increased cost, and worse quality of life. As many psychosocial risk factors are potentially modifiable, at-risk patients undergoing HSCT would be an ideal population for a prospective study with targeted interventions such as early utilization of cancer psychologists, counselors, or healthcare navigators. Further research needs to be pursued to delineate which, if any, particular psychosocial risk factors are driving this association.
Andritsos:Hairy Cell Leukemia Foundation: Research Funding. Hofmeister:Arno Therapeutics, Inc.: Research Funding; Signal Genetics, Inc.: Membership on an entity's Board of Directors or advisory committees; Janssen: Pharmaceutical Companies of Johnson & Johnson: Research Funding; Incyte, Corp: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Karyopharm Therapeutics: Research Funding; Takeda Pharmaceutical Company: Research Funding; Teva: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal