Abstract
Background. Haploidentical bone marrow transplantation (HAPLO-BMT) with post-transplant cyclophosphamide (PT-CY) is being increasingly used in patients with acute myeloid leukemia (AML) who lack a suitable HLA-matched donor. The standard Baltimore regimen calls for PT-CY 50 mg/kg on days +3 and +4, with a calcineurin inhibitor and mycophenolate (MMF) starting on day +5 after transplant.
Aim of the study. We have modified the original Baltimore regimen (BBMT 2013; 19:117), and are now reporting a multicenter retrospective analysis of HAPLO-BMT in 142 patients with AML.
GvHD prophylaxis. All patients received a uniform GvHD prophylaxis , consisting of cyclosporine (CsA) starting on day 0, mycophenolate (MMF) starting on day +1, and PT-CY 50 mg/kg , on days +3 and +5.
Patients and conditioning regimen. All patients received umanipulated haploidentical marrow between year 2010 and 2016. Clinical characteristics included: 73 males and 69 females, median age of 50 years (17-74); low ELN risk group (3%) intermediate risk (34%) and high risk (63%); FLT3-ITD positivity (22%) ; first complete remission (CR1) (46%) , second CR (CR2) (21%) and active disease (33%). The median dose of TNC collected and infused was 3,1x108/kg (range 0,8-6,5). All patients received a myeloablative regimen: either thiotepa (10 mg/kg), busulfan (3.2 mg/kgx3), fludarabine (50 mg/m^2x3) (TBF) in 114 patients (median age 55 years), or full dose TBI in 28 patients (median age 37 years). Busulfan was capped at 2 days in patients over 60 years. The median follow up for surviving patients is 532 days (100-1893)
Results. 133 patients (94%) engrafted; the median interval to a neutrophil count of 0,5x10^9/L was day 18 (range 13-56). The 100 day cumulative incidence (CI) of grade II-IV and III-IV aGVHD was 17% and 3%. Chronic GVHD was observed in 65 patients with a cumulative incidence of moderate and severe cGVHD of 16% at 3 years.
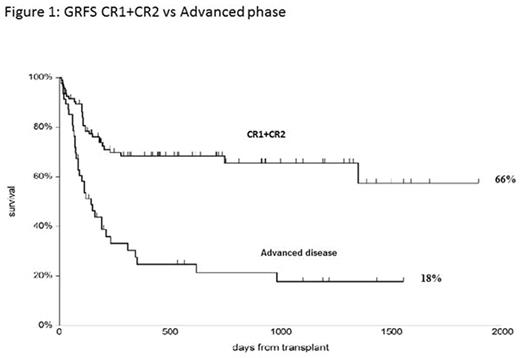
The cumulative incidence of transplant related mortality (TRM) at 5 years is 18%; the CI of relapse related death (RRD) is 32%. Causes of death were relapse (n=26), infections (n=14), graft failure (n=3), multi-organ failure (n=2) chronic GvHD (n=2) , and interstitial pneumonia (n=2). Patients in CR1, CR2, or with active disease, have an actuarial 5 year overall survival (OS) of 79%, 67% and 22%, respectively (p<0,00001); a CI of TRM at 5 years of 11%, 20% and 28% (p=0,05) and a RRD of 8%, 10% and 50% respectively (p<0,00001). The OS according to risk groups is 78%, for intermediate risk and 48% for high risk patients (p=0,001). Survival free of GvHD and relapse (GRFS) at 5 years, is 66% for CR1+CR2 patients and 18% for pts with advanced disease (Fig.1).
In multivariate Cox analysis, active disease at transplant is the only negative predictor of survival. Despite older age of patients given TBF (almost 20 year difference), survival is comparable to the TBI regimen: the OS of 22 patients aged 60 and over, receiving TBF, grafted in CR1 or CR2, is 82%.
Center effect. There was no significant Center effect and the actuarial 5 year OS for CR1+CR2 patients grafted in Genova (n=68) or outside Genova (n=27) was identical (75%).
Conclusion. This study shows that our modified PT-CY regimen, with CyA given before PT-CY, and one day rest between the two CY doses, can be successfully applied in a multicenter setting of unmanipulated HAPLO-BMT for AML. For CR1,CR2 patients the outcome is excellent in terms of TRM, RRD and survival, particularly with the TBF conditioning, also in patients over 60. Relapse remains a problem in patients with active disease, as seen with any conventional transplant platform, and may require post-transplant interventions. The incidence of severe acute and chronic GvHD is very low, with marrow as the only stem cell source for all patients.
Bug:Novartis: Honoraria, Research Funding; Nord Medica: Consultancy; Celgene: Honoraria, Other: Travel Grant; Janssen: Other: Travel Grant; Astellas: Other: Travel Grant; Teva Oncology: Other: Travel Grant. Angelucci:Novartis oncology, celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal