Abstract
Introduction. Haploidentical donors may resolve the shortage of available HLA-matched donors for the treatment of patients with blood cancers in need of a hematopoietic stem cell transplantation (HSCT). However, to prevent graft-versus-host disease (GVHD), haploidentical HSCT requires alloreactive T-cell depletion or suppression. We developed an ex vivo cell treatment strategy that allows additional donor lymphocytes to be infused post-HSCT without inducing severe GVHD and maintaining reactivity against viruses and leukemic cells.
Patients and Methods. In this open-label, multicenter phase 2 study (CR-AIR-007; NCT01794299), 23 patients, 11 females and 12 males, with a median age of 41 years (range: 21 - 64) were treated with ATIR101. Sixteen patients had AML and seven patients had ALL (15 in CR1 and 8 in CR2). Patients underwent myeloablative conditioning, consisting of a] TBI (1200 cGy: n=11) or b] melphalan (120 mg/m2: n=12), along with thiotepa (10 mg/kg), fludarabine (30 mg/m2 x 5d) and ATG (2.5mg/kg x 4d). A CD34+ selected stem cell graft from a haploidentical donor was given, containing a mean of 11x106 CD34+ cells/kg (range: 4.7 - 24.4x106) and 0.29x104 CD3+ cells/kg (range: 0 - 1.8x104). In addition, donor lymphocytes from the same donor were processed using a selective photodepletion technology, creating a donor lymphocyte infusion depleted of alloreactive T-cells (ATIR101). ATIR101 was infused at a median of 28 days (range: 28-73) post-HSCT at a fixed dose of 2x106 CD3+ cells/kg, without use of any post-transplant GVHD prophylaxis.
Results. All patients engrafted rapidly after transplantation, with neutrophil and platelet engraftment achieved at a median of 12 days (range: 8-34 and 9-35, respectively). Median follow-up (as of August 1st, 2016) is 484 days post-HSCT, with at least 1-year follow-up for all but one patient, which will reach the 1 year endpoint end of September 2016. Ex vivo photodepletion eliminated anti-host proliferating T cells while preserving anti-third party and anti-CD3/CD28 reactivity. CD3+ T-cells were detected in patient peripheral blood from 16 weeks post-HSCT and CD19+ B cells increased rapidly from 5 weeks post-HSCT onwards. No patients developed grade III/IV acute GVHD after infusion of ATIR101. Three cases of grade II acute GVHD were reported and only one case of chronic GVHD has been reported so far. No patient died within 100 days post-HSCT and the overall survival of patients given ATIR101 was significantly improved compared to a historic control group, with a 1-year survival of 61% in the HSCT+ATIR101 group vs 20% in the control group (Table 1). Thus far two patients experienced a relapse within the first year post-HSCT, occurring at 60 and 90 days, respectively. Severe infections (grade 3-5) occurred in 9/23 subjects within the first 6 months post-HSCT, which were viral infections in 7/23 subjects. After 6 months, up to a year post-HSCT only 5/23 patients were reported to suffer from severe infections.
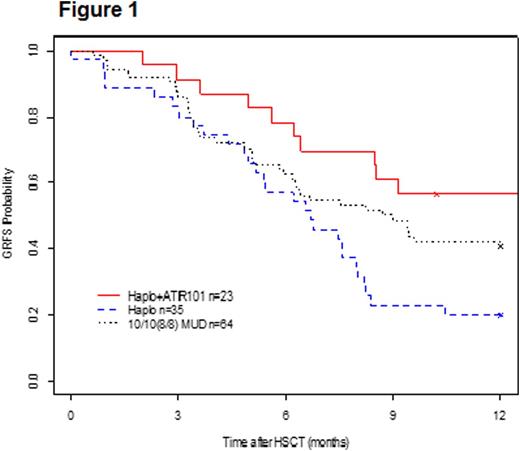
The GVHD-free, relapse-free survival (GRFS) for HSCT + ATIR101 is estimated to be 57% at 1-year post-HSCT, which compares favorably to the T-cell depleted HSCT only control group (20%), and also against the MUD group (41%), derived from the same centers in these contemporaneous historical control groups (Figure 1).
Conclusion. Administration of a high dose of donor lymphocytes in ATIR101 from a haploidentical donor does not cause severe GVHD without use of prophylactic immune suppression. Addition of ATIR101 to a T-cell depleted HSCT protocol significantly improves transplantation outcome, with improved overall survival and favorable event-free survival (GRFS). One year GRFS for HSCT + ATIR101 also compares favorably to published data on haploidentical HSCT using post-transplant cyclophosphamide for GVHD prophylaxis (Solh et al, BBMT 2016).
Roy:Kiadis Pharma: Research Funding; Novartis: Consultancy; Fate Therapeutics: Consultancy; Paladin: Consultancy. Maertens:Astellas: Consultancy, Speakers Bureau; Merck Sharp & Dohme: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy; Gilead: Consultancy, Honoraria, Speakers Bureau. Rüdiger:Kiadis Pharma: Employment. Velthuis:Kiadis Pharma: Employment. Gerez:Kiadis Pharma: Employment. Rovers:Kiadis Pharma: Employment. Mielke:JAZZ Pharma: Speakers Bureau; Novartis: Consultancy; Gilead: Other: Travel grants; Celgene: Other: Travel grants, Speakers Bureau; MSD: Consultancy, Other: Travel grants.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal